Moving forward: advances in the treatment of movement disorders with deep brain stimulation
- PMID: 22084629
- PMCID: PMC3211039
- DOI: 10.3389/fnint.2011.00069
Moving forward: advances in the treatment of movement disorders with deep brain stimulation
Abstract
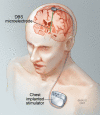
The modern era of stereotactic and functional neurosurgery has ushered in state of the art technologies for the treatment of movement disorders, particularly Parkinson's disease (PD), tremor, and dystonia. After years of experience with various surgical therapies, the eventual shortcomings of both medical and surgical treatments, and several serendipitous discoveries, deep brain stimulation (DBS) has risen to the forefront as a highly effective, safe, and reversible treatment for these conditions. Idiopathic advanced PD can be treated with thalamic, globus pallidus internus (GPi), or subthalamic nucleus (STN) DBS. Thalamic DBS primarily relieves tremor while GPi and STN DBS alleviate a wide range of Parkinsonian symptoms. Thalamic DBS is also used in the treatment of other types of tremor, particularly essential tremor, with excellent results. Both primary and various types of secondary dystonia can be treated very effectively with GPi DBS. The variety of anatomical targets for these movement disorders is indicative of the network-level dysfunction mediating these movement disturbances. Despite an increasing understanding of the clinical benefits of DBS, little is known about how DBS can create such wide sweeping neuromodulatory effects. The key to improving this therapeutic modality and discovering new ways to treat these and other neurologic conditions lies in better understanding the intricacies of DBS. Here we review the history and pertinent clinical data for DBS treatment of PD, tremor, and dystonia. While multiple regions of the brain have been targeted for DBS in the treatment of these movement disorders, this review article focuses on those that are most commonly used in current clinical practice. Our search criteria for PubMed included combinations of the following terms: DBS, neuromodulation, movement disorders, PD, tremor, dystonia, and history. Dates were not restricted.
Keywords: Parkinson’s disease; deep brain stimulation; dystonia; neuromodulation; tremor.
Figures


References
-
- Agnesi F., Tye S. J., Bledsoe J. M., Griessenauer C. J., Kimble C. J., Sieck G. C., Bennet K. E., Garris P. A., Blaha C. D., Lee K. H. (2009). Wireless instantaneous neurotransmitter concentration system-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J. Neurosurg. 111, 701–71110.3171/2009.3.JNS0990 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

