An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury
- PMID: 22085254
- PMCID: PMC3411361
- DOI: 10.1089/scd.2011.0596
An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury
Abstract
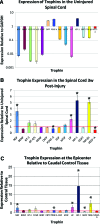
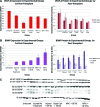
Cellular transplantation strategies for repairing the injured spinal cord have shown consistent benefit in preclinical models, and human clinical trials have begun. Interactions between transplanted cells and host tissue remain poorly understood. Trophic factor secretion is postulated a primary or supplementary mechanism of action for many transplanted cells, however, there is little direct evidence to support trophin production by transplanted cells in situ. In the present study, trophic factor expression was characterized in uninjured, injured-untreated, injured-treated with transplanted cells, and corresponding control tissue from the adult rat spinal cord. Candidate trophic factors were identified in a literature search, and primers were designed for these genes. We examined in vivo trophin expression in 3 paradigms involving transplantation of either brain or spinal cord-derived neural precursor cells (NPCs) or bone marrow stromal cells (BMSCs). Injury without further treatment led to a significant elevation of nerve growth factor (NGF), leukemia inhibitory factor (LIF), insulin-like growth factor-1 (IGF-1), and transforming growth factor-β1 (TGF-β1), and lower expression of vascular endothelial growth factor isoform A (VEGF-A) and platelet-derived growth factor-A (PDGF-A). Transplantation of NPCs led to modest changes in trophin expression, and the co-administration of intrathecal trophins resulted in significant elevation of the neurotrophins, glial-derived neurotrophic factor (GDNF), LIF, and basic fibroblast growth factor (bFGF). BMSCs transplantation upregulated NGF, LIF, and IGF-1. NPCs isolated after transplantation into the injured spinal cord expressed the neurotrophins, ciliary neurotrophic factor (CNTF), epidermal growth factor (EGF), and bFGF at higher levels than host cord. These data show that trophin expression in the spinal cord is influenced by injury and cell transplantation, particularly when combined with intrathecal trophin infusion. Trophins may contribute to the benefits associated with cell-based repair strategies for spinal cord injury.
Figures




References
-
- Hawryluk GW. Fehlings MG. Cellular transplantation approaches for repair of the injured spinal cord: ongoing and pending clinical trials. Top SCI Rehabil. 2009;14:47–61.
-
- Pearse DD. Pereira FC. Marcillo AE. Bates ML. Berrocal YA. Filbin MT. Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. - PubMed
-
- Erceg S. Ronaghi M. Oria M. Rosello MG. Arago MA. Lopez MG. Radojevic I. Moreno-Manzano V. Rodriguez-Jimenez FJ, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. - PMC - PubMed
-
- Enzmann GU. Benton RL. Talbott JF. Cao Q. Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23:479–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
