Efficacy and safety of inhaled formoterol 4.5 and 9 μg twice daily in Japanese and European COPD patients: phase III study results
- PMID: 22085439
- PMCID: PMC3233513
- DOI: 10.1186/1471-2466-11-51
Efficacy and safety of inhaled formoterol 4.5 and 9 μg twice daily in Japanese and European COPD patients: phase III study results
Abstract
Background: This study evaluated the efficacy and safety of the long-acting β₂-agonist formoterol in patients with moderate-to-severe COPD.
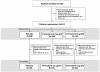
Methods: This double-blind, placebo-controlled, parallel-group, multinational phase III study randomized patients ≥ 40 years of age with moderate-to-severe COPD to inhaled formoterol 4.5 or 9 μg twice daily (bid) via Turbuhaler or placebo for 12 weeks. Salbutamol 100 μg/actuation via pMDI was permitted as reliever medication. The primary outcome variable was change (ratio) from baseline to treatment period in FEV1 60-min post-dose.
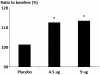
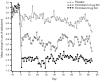
Results: 613 patients received treatment (formoterol 4.5 μg n = 206; 9 μg n = 199; placebo n = 208); 539 (87.9%) male; 324 (52.9%) Japanese and 289 (47.1%) European. End of study increases in FEV1 60-min post-dose were significantly greater (p < 0.001 for both) with formoterol 4.5 and 9 μg bid (113% of baseline for both) than with placebo, as were all secondary outcome measures. The proportion of patients with an improvement in St George's Respiratory Questionnaire score of ≥ 4 was 50.2% for formoterol 4.5 μg (p = 0.0682 vs. placebo), 59.2% (p = 0.0004) for 9 μg, and 41.3% for placebo. Reduction in reliever medication use was significantly greater with formoterol vs. placebo (9 μg: -0.548, p < 0.001; 4.5 μg: -0.274, p = 0.027), with 9 μg being significantly superior to 4.5 μg (-0.274, p = 0.029). Formoterol was well tolerated with the incidence and type of adverse events not being different for the three groups.
Conclusions: Formoterol 4.5 μg and 9 μg bid was effective and well tolerated in patients with COPD; there was no difference between formoterol doses for the primary endpoint; however, an added value of formoterol 9 μg over 4.5 μg bid was observed for some secondary endpoints.
Trial registration: NCT00628862 (ClinicalTrials.gov); D5122C00001 (AstraZeneca Study code).
Figures
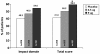
References
-
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–1999. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

