Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells
- PMID: 22085487
- PMCID: PMC3315001
- DOI: 10.1016/j.heares.2011.10.008
Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells
Abstract
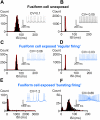
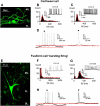
Acoustic over-exposure (AOE) triggers deafness in animals and humans and provokes auditory nerve degeneration. Weeks after exposure there is an increase in the cellular excitability within the dorsal cochlear nucleus (DCN) and this is considered as a possible neural correlate of tinnitus. The origin of this DCN hyperactivity phenomenon is still unknown but it is associated with neurons lying within the fusiform cell layer. Here we investigated changes of excitability within identified fusiform cells following AOE. Wistar rats were exposed to a loud (110 dB SPL) single tone (14.8 kHz) for 4 h. Auditory brainstem response recordings performed 3-4 days after AOE showed that the hearing thresholds were significantly elevated by about 20-30 dB SPL for frequencies above 15 kHz. Control fusiform cells fired with a regular firing pattern as assessed by the coefficient of variation of the inter-spike interval distribution of 0.19 ± 0.11 (n = 5). Three to four days after AOE, 40% of fusiform cells exhibited irregular bursting discharge patterns (coefficient of variation of the inter-spike interval distribution of 1.8 ± 0.6, n = 5; p < 0.05). Additionally the maximal firing following step current injections was reduced in these cells (from 83 ± 11 Hz, n = 5 in unexposed condition to 43 ± 6 Hz, n = 5 after AOE) and this was accompanied by an increased firing gain (from 0.09 ± 0.01 Hz/pA, n = 5 in unexposed condition to 0.56 ± 0.25 Hz/pA, n = 5 after AOE). Current and voltage clamp recordings suggest that the presence of bursts in fusiform cells is related to a down regulation of high voltage activated potassium currents. In conclusion we showed that AOE triggers deafness at early stages and this is correlated with profound changes in the firing pattern and frequency of the DCN major output fusiform cells. The changes here described could represent the initial network imbalance prior to the emergence of tinnitus.
Crown Copyright © 2011. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Barnes-Davies M., Barker M.C., Osmani F., Forsythe I.D. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur. J. Neurosci. 2004;19:325–333. - PubMed
-
- Barrs D.M., Brackmann D.E., Hitselberger W.E. Facial nerve anastomosis in the cerebellopontine angle: a review of 24 cases. Am. J. Otol. 1984;5:269–272. - PubMed
-
- Buzsaki G., Csicsvari J., Dragoi G., Harris K., Henze D., Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb. Cortex. 2002;12:893–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

