Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy
- PMID: 22085543
- PMCID: PMC4545509
- DOI: 10.1016/j.exger.2011.10.011
Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy
Abstract
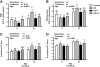
In the present study, we investigated the effects of 7 and 14 days of re-loading following 14-day muscle unweighting (hindlimb suspension, HS) on iron transport, non-heme iron levels and oxidative damage in the gastrocnemius muscle of young (6 months) and old (32 months) male Fischer 344×Brown Norway rats. Our results demonstrated that old rats had lower muscle mass, higher levels of total non-heme iron and oxidative damage in skeletal muscle in comparison with young rats. Non-heme iron concentrations and total non-heme iron amounts were 3.4- and 2.3-fold higher in aged rats as compared with their young counterparts, respectively. Seven and 14 days of re-loading was associated with higher muscle weights in young animals as compared with age-matched HS rats, but there was no difference in muscle weights among aged HS, 7 and 14 days of re-loading rats, indicating that aged rats may have a lower adaptability to muscle disuse and a lower capacity to recover from muscle atrophy. Protein levels of cellular iron transporters, such as divalent metal transport-1 (DMT1), transferrin receptor-1 (TfR1), Zip14, and ferroportin (FPN), and their mRNA abundance were determined. TfR1 protein and mRNA levels were significantly lower in aged muscle. Seven and 14 days of re-loading were associated with higher TfR1 mRNA and protein levels in young animals in comparison with their age-matched HS counterparts, but there was no difference between cohorts in aged animals, suggesting adaptive responses in the old to cope with iron deregulation. The extremely low expression of FPN in skeletal muscle might lead to inefficient iron export in the presence of iron overload and play a critical role in age-related iron accumulation in skeletal muscle. Moreover, oxidative stress was much greater in the muscles of the older animals measured as 4-hydroxy-2-nonhenal (HNE)-modified proteins and 8-oxo-7,8-dihydroguanosine levels. These markers remained fairly constant with either HS or re-loading in young rats. In old rats, HNE-modified proteins and 8-oxo-7,8-dihydroguanosine levels were markedly higher in HS and were lower after 7 days of recovery. However, no difference was observed following 14 days of recovery between control and re-loading animals. In conclusion, advanced age is associated with disruption of muscle iron metabolism which is further perturbed by disuse and persists over a longer time period.
Published by Elsevier Inc.
Figures




References
-
- Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–233. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44:413–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

