Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis
- PMID: 22085732
- PMCID: PMC3388653
- DOI: 10.1186/cc10545
Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis
Abstract
Introduction: The optimal duration of antibiotic therapy for bloodstream infections is unknown. Shorter durations of therapy have been demonstrated to be as effective as longer durations for many common infections; similar findings in bacteremia could enable hospitals to reduce antibiotic utilization, adverse events, resistance and costs.
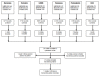
Methods: A search of the MEDLINE, EMBASE and COCHRANE databases was conducted for the years 1947-2010. Controlled trials were identified that randomized patients to shorter versus longer durations of treatment for bacteremia, or the infectious foci most commonly causing bacteremia in critically ill patients (catheter-related bloodstream infections (CRBSI), intra-abdominal infections, pneumonia, pyelonephritis and skin and soft-tissue infections (SSTI)).
Results: Twenty-four eligible trials were identified, including one trial focusing exclusively on bacteremia, zero in catheter related bloodstream infection, three in intra-abdominal infection, six in pyelonephritis, thirteen in pneumonia and one in skin and soft tissue infection. Thirteen studies reported on 227 patients with bacteremia allocated to 'shorter' or 'longer' durations of treatment. Outcome data were available for 155 bacteremic patients: neonatal bacteremia (n = 66); intra-abdominal infection (40); pyelonephritis (9); and pneumonia (40). Among bacteremic patients receiving shorter (5-7 days) versus longer (7-21 days) antibiotic therapy, no significant difference was detected with respect to rates of clinical cure (45/52 versus 47/49, risk ratio 0.88, 95% confidence interval [CI] 0.77-1.01), microbiologic cure (28/28 versus 30/32, risk ratio 1.05, 95% CI 0.91-1.21), and survival (15/17 versus 26/29, risk ratio 0.97, 95% CI 0.76-1.23).
Conclusions: No significant differences in clinical cure, microbiologic cure and survival were detected among bacteremic patients receiving shorter versus longer duration antibiotic therapy. An adequately powered randomized trial of bacteremic patients is needed to confirm these findings.
Figures
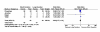
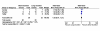
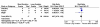
Comment in
-
Duration of antibiotic therapy in bacteraemia.Crit Care. 2012 Jan 9;16(1):403. doi: 10.1186/cc10590. Crit Care. 2012. PMID: 22236377 Free PMC article. No abstract available.
References
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. - DOI - PubMed
-
- Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. - PubMed
-
- Renaud B, Brun-Buisson C. Outcomes of primary and catheter-related bacteremia. Am J Respir Crit Care Med. 2001;163:1584–1590. - PubMed
-
- Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, De Lassence A, Azoulay E, Cheval C, Descorps-Declere A, Adrie C, Costa de Beauregard MA, Carlet J. OUTCOMEREA Study Group. Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis. 2006;42:1118–1126. doi: 10.1086/500318. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

