Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome
- PMID: 22085958
- PMCID: PMC3280938
- DOI: 10.1186/1477-5956-9-71
Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome
Abstract
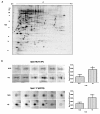
Background: The effects of fetal calf serum (FCS) heat inactivation and bacterial lipopolysaccharide (LPS) contamination on cell physiology have been studied, but their effect on the proteome of cultured cells has yet to be described. This study was undertaken to investigate the effects of heat inactivation of FCS and LPS contamination on the human T lymphoblast proteome. Human T lymphoblastic leukaemia (CCRF-CEM) cells were grown in FCS, either non-heated, or heat inactivated, having low (< 1 EU/mL) or regular (< 30 EU/mL) LPS concentrations. Protein lysates were resolved by 2-DE followed by phospho-specific and silver nitrate staining. Differentially regulated spots were identified by nano LC ESI Q-TOF MS/MS analysis.
Results: A total of four proteins (EIF3M, PRS7, PSB4, and SNAPA) were up-regulated when CCRF-CEM cells were grown in media supplemented with heat inactivated FCS (HE) as compared to cells grown in media with non-heated FCS (NHE). Six proteins (TCPD, ACTA, NACA, TCTP, ACTB, and ICLN) displayed a differential phosphorylation pattern between the NHE and HE groups. Compared to the low concentration LPS group, regular levels of LPS resulted in the up-regulation of three proteins (SYBF, QCR1, and SUCB1).
Conclusion: The present study provides new information regarding the effect of FCS heat inactivation and change in FCS-LPS concentration on cellular protein expression, and post-translational modification in human T lymphoblasts. Both heat inactivation and LPS contamination of FCS were shown to modulate the expression and phosphorylation of proteins involved in basic cellular functions, such as protein synthesis, cytoskeleton stability, oxidative stress regulation and apoptosis. Hence, the study emphasizes the need to consider both heat inactivation and LPS contamination of FCS as factors that can influence the T lymphoblast proteome.
Figures


Similar articles
-
Differential proteome and phosphoproteome signatures in human T-lymphoblast cells induced by sirolimus.Cell Prolif. 2010 Aug;43(4):396-404. doi: 10.1111/j.1365-2184.2010.00690.x. Cell Prolif. 2010. PMID: 20590665 Free PMC article.
-
Serum-mediated modification of proliferation in factor-dependent macrophage cell lines.Cell Struct Funct. 1993 Aug;18(4):211-9. doi: 10.1247/csf.18.211. Cell Struct Funct. 1993. PMID: 8293498
-
Heat inactivation of fetal calf serum is not required for in vitro measurement of lymphocyte functions.J Immunol Methods. 1999 Mar 4;223(2):249-54. doi: 10.1016/s0022-1759(98)00214-2. J Immunol Methods. 1999. PMID: 10089103
-
No evidence for αGal epitope transfer from media containing FCS onto human endothelial cells in culture.Xenotransplantation. 2015 Sep-Oct;22(5):345-55. doi: 10.1111/xen.12183. Epub 2015 Aug 24. Xenotransplantation. 2015. PMID: 26301779
-
Mouse serum as a medium supplement for murine immune responses in vitro.J Immunol Methods. 1985 Jan 21;76(1):17-26. doi: 10.1016/0022-1759(85)90477-6. J Immunol Methods. 1985. PMID: 3871461
Cited by
-
Identification of the novel interacting partners of the mammalian target of rapamycin complex 1 in human CCRF-CEM and HEK293 cells.Int J Mol Sci. 2014 Mar 18;15(3):4823-36. doi: 10.3390/ijms15034823. Int J Mol Sci. 2014. PMID: 24646917 Free PMC article.
-
Development, Establishment, and Validation of a Model for the Mineralization of Periodontium Remodelling Cells: Cementoblasts.Int J Mol Sci. 2023 Sep 7;24(18):13829. doi: 10.3390/ijms241813829. Int J Mol Sci. 2023. PMID: 37762132 Free PMC article.
-
Effects of Adipose-Derived Stem Cells and Their Conditioned Medium in a Human Ex Vivo Wound Model.Cells. 2022 Apr 2;11(7):1198. doi: 10.3390/cells11071198. Cells. 2022. PMID: 35406762 Free PMC article.
-
Effect of fetal bovine serum on foamy and lentiviral vector production.Hum Gene Ther Methods. 2013 Oct;24(5):307-9. doi: 10.1089/hgtb.2013.097. Epub 2013 Aug 28. Hum Gene Ther Methods. 2013. PMID: 23984723 Free PMC article.
-
Probing the prostate tumour microenvironment I: impact of glucose deprivation on a cell model of prostate cancer progression.Oncotarget. 2017 Feb 28;8(9):14374-14394. doi: 10.18632/oncotarget.14605. Oncotarget. 2017. PMID: 28086232 Free PMC article.
References
-
- Goustin AS, Leof EB, Shipley GD, Moses HL. Growth factors and cancer. Cancer Res. 1986;46:1015–1029. - PubMed
-
- Morgan JF, Morton HJ, Parker RC. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950;73:1–8. - PubMed
-
- Barta O, Nelson RA, Kuo CY. Separation of six bovine complement components and one inactivator (1, 2) Immunol Commun. 1976;5:75–86. - PubMed
-
- Kimura T, Miyazaki K, Mashima K, Yamashita J, Horio T, Kakuno T. Purification and properties of growth inhibitor from normal rabbit serum. J Biochem. 1991;110:423–428. - PubMed
-
- Otsuka H. An inhibitor present in calf serum which prevents growth of BHK21 cells in suspension culture. J Cell Sci. 1972;10:137–152. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

