Maelstrom coordinates microtubule organization during Drosophila oogenesis through interaction with components of the MTOC
- PMID: 22085963
- PMCID: PMC3222902
- DOI: 10.1101/gad.174110.111
Maelstrom coordinates microtubule organization during Drosophila oogenesis through interaction with components of the MTOC
Abstract
The establishment of body axes in multicellular organisms requires accurate control of microtubule polarization. Mutations in Drosophila PIWI-interacting RNA (piRNA) pathway genes often disrupt the axes of the oocyte. This results from the activation of the DNA damage checkpoint factor Checkpoint kinase 2 (Chk2) due to transposon derepression. A piRNA pathway gene, maelstrom (mael), is critical for the establishment of oocyte polarity in the developing egg chamber during Drosophila oogenesis. We show that Mael forms complexes with microtubule-organizing center (MTOC) components, including Centrosomin, Mini spindles, and γTubulin. We also show that Mael colocalizes with αTubulin and γTubulin to centrosomes in dividing cyst cells and follicle cells. MTOC components mislocalize in mael mutant germarium and egg chambers, leading to centrosome migration defects. During oogenesis, the loss of mael affects oocyte determination and induces egg chamber fusion. Finally, we show that the axis specification defects in mael mutants are not suppressed by a mutation in mnk, which encodes a Chk2 homolog. These findings suggest a model in which Mael serves as a platform that nucleates other MTOC components to form a functional MTOC in early oocyte development, which is independent of Chk2 activation and DNA damage signaling.
Figures

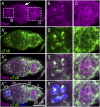
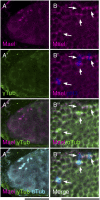
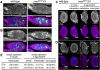
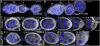
References
-
- Aravin AA, Naumova NM, Tulin AV, Vagin VV, Rozovsky YM, Gvozdev VA 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr Biol 11: 1017–1027 - PubMed
-
- Arn EA, Cha BJ, Theurkauf WE, Macdonald PM 2003. Recognition of a bicoid mRNA localization signal by a protein complex containing Swallow, Nod, and RNA binding proteins. Dev Cell 4: 41–51 - PubMed
-
- Bolívar J, Huynh JR, López-Schier H, González C, St Johnston D, González-Reyes A 2001. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development 128: 1889–1897 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
