Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression
- PMID: 22085964
- PMCID: PMC3222903
- DOI: 10.1101/gad.178079.111
Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression
Abstract
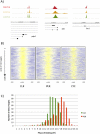
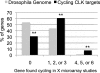
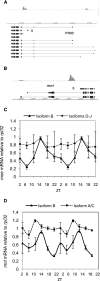
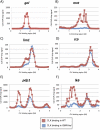
CLOCK (CLK) is a master transcriptional regulator of the circadian clock in Drosophila. To identify CLK direct target genes and address circadian transcriptional regulation in Drosophila, we performed chromatin immunoprecipitation (ChIP) tiling array assays (ChIP-chip) with a number of circadian proteins. CLK binding cycles on at least 800 sites with maximal binding in the early night. The CLK partner protein CYCLE (CYC) is on most of these sites. The CLK/CYC heterodimer is joined 4-6 h later by the transcriptional repressor PERIOD (PER), indicating that the majority of CLK targets are regulated similarly to core circadian genes. About 30% of target genes also show cycling RNA polymerase II (Pol II) binding. Many of these generate cycling RNAs despite not being documented in prior RNA cycling studies. This is due in part to different RNA isoforms and to fly head tissue heterogeneity. CLK has specific targets in different tissues, implying that important CLK partner proteins and/or mechanisms contribute to gene-specific and tissue-specific regulation.
Figures


Comment in
-
A master CLOCK hard at work brings rhythm to the transcriptome.Genes Dev. 2011 Nov 15;25(22):2321-6. doi: 10.1101/gad.180984.111. Genes Dev. 2011. PMID: 22085960 Free PMC article.
References
-
- Allada R, White N, So W, Hall J, Rosbash M 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93: 791–804 - PubMed
-
- Blau J, Young MW 1999. Cycling vrille expression is requiered for a functional Drosophila clock. Cell 99: 661–671 - PubMed
-
- Curtin K, Huang ZJ, Rosbash M 1995. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 14: 365–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
