Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea
- PMID: 22086147
- PMCID: PMC3254718
- DOI: 10.1007/s10162-011-0297-9
Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea
Abstract
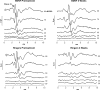
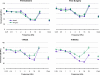
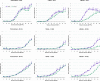
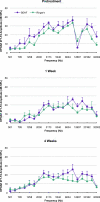
Neurotrophins prevent spiral ganglion neuron (SGN) degeneration in animal models of ototoxin-induced deafness and may be used in the future to improve the hearing of cochlear implant patients. It is increasingly common for patients with residual hearing to undergo cochlear implantation. However, the effect of neurotrophin treatment on acoustic hearing is not known. In this study, brain-derived neurotrophic factor (BDNF) was applied to the round window membrane of adult guinea pigs for 4 weeks using a cannula attached to a mini-osmotic pump. SGN survival was first assessed in ototoxically deafened guinea pigs to establish that the delivery method was effective. Increased survival of SGNs was observed in the basal and middle cochlear turns of deafened guinea pigs treated with BDNF, confirming that delivery to the cochlea was successful. The effects of BDNF treatment in animals with normal hearing were then assessed using distortion product otoacoustic emissions (DPOAEs), pure tone, and click-evoked auditory brainstem responses (ABRs). DPOAE assessment indicated a mild deficit of 5 dB SPL in treated and control groups at 1 and 4 weeks after cannula placement. In contrast, ABR evaluation showed that BDNF lowered thresholds at specific frequencies (8 and 16 kHz) after 1 and 4 weeks posttreatment when compared to the control cohort receiving Ringer's solution. Longer treatment for 4 weeks not only widened the range of frequencies ameliorated from 2 to 32 kHz but also lowered the threshold by at least 28 dB SPL at frequencies ≥16 kHz. BDNF treatment for 4 weeks also increased the amplitude of the ABR response when compared to either the control cohort or prior to treatment. We show that BDNF applied to the round window reduces auditory thresholds and could potentially be used clinically to protect residual hearing following cochlear implantation.
Figures





References
-
- Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Tena JJ, Benitez-Temino B, Pecero ML, Morcuende SR, Cruz RR, Pastor AM. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci. 2009;29:575–587. doi: 10.1523/JNEUROSCI.5312-08.2009. - DOI - PMC - PubMed
-
- Diao MF, Gao WY, Sun JJ, Liu Y, Chen DL, Jiang W, Zhao J, Chen X. Protection from noise-induced hearing loss by a nitric oxide synthase inhibitor and neurotrophin 3 in the guinea pig cochlea. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:281–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

