Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells
- PMID: 22086415
- PMCID: PMC3257008
- DOI: 10.1182/blood-2011-05-353789
Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells
Abstract
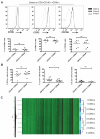
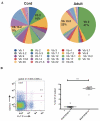
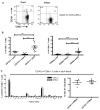
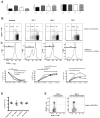
Human mucosal associated invariant T (MAIT) CD8(+) and Tc17 cells are important tissue-homing cell populations, characterized by high expression of CD161 ((++)) and type-17 differentiation, but their origins and relationships remain poorly defined. By transcriptional and functional analyses, we demonstrate that a pool of polyclonal, precommitted type-17 CD161(++)CD8αβ(+) T cells exist in cord blood, from which a prominent MAIT cell (TCR Vα7.2(+)) population emerges post-natally. During this expansion, CD8αα T cells appear exclusively within a CD161(++)CD8(+)/MAIT subset, sharing cytokine production, chemokine-receptor expression, TCR-usage, and transcriptional profiles with their CD161(++)CD8αβ(+) counterparts. Our data demonstrate the origin and differentiation pathway of MAIT-cells from a naive type-17 precommitted CD161(++)CD8(+) T-cell pool and the distinct phenotype and function of CD8αα cells in man.
Figures



References
-
- Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. - PubMed
-
- Annibali V, Ristori G, Angelini DF, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134(Pt 2):542–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

