Contrasting dynamic responses in vivo of the Bcl-xL and Bim erythropoietic survival pathways
- PMID: 22086418
- PMCID: PMC3277355
- DOI: 10.1182/blood-2011-07-365346
Contrasting dynamic responses in vivo of the Bcl-xL and Bim erythropoietic survival pathways
Abstract
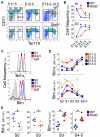
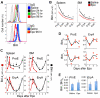
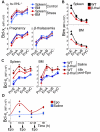
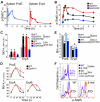
Survival signaling by the erythropoietin (Epo) receptor (EpoR) is essential for erythropoiesis and for its acceleration in hypoxic stress. Several apparently redundant EpoR survival pathways were identified in vitro, raising the possibility of their functional specialization in vivo. Here we used mouse models of acute and chronic stress, including a hypoxic environment and β-thalassemia, to identify two markedly different response dynamics for two erythroblast survival pathways in vivo. Induction of the antiapoptotic protein Bcl-x(L) is rapid but transient, while suppression of the proapoptotic protein Bim is slower but persistent. Similar to sensory adaptation, however, the Bcl-x(L) pathway "resets," allowing it to respond afresh to acute stress superimposed on a chronic stress stimulus. Using "knock-in" mouse models expressing mutant EpoRs, we found that adaptation in the Bcl-x(L) response occurs because of adaptation of its upstream regulator Stat5, both requiring the EpoR distal cytoplasmic domain. We conclude that survival pathways show previously unsuspected functional specialization for the acute and chronic phases of the stress response. Bcl-x(L) induction provides a "stop-gap" in acute stress, until slower but permanent pathways are activated. Furthermore, pathologic elevation of Bcl-x(L) may be the result of impaired adaptation, with implications for myeloproliferative disease mechanisms.
Figures



References
-
- Erslev AJ, Caro J. Erythropoietin titers in response to anemia or hypoxia. Blood Cells. 1987;13(1-2):207–216. - PubMed
-
- Lodish HF, Ghaffari S, Socolovsky M, Tong W, Zhang J. Intracellular signaling by the erythropoietin receptor. In: Elliott SG, Foote M, Molineux G, editors. Erythropoietins, Erythropoietic Factors, and Erythropoiesis: Molecular, Cellular, Preclinical, and Clinical Biology. Basel, Switzerland: Birkhäuser; 2009. pp. 155–174.
-
- Koury MJ, Bondurant MC. The molecular mechanism of erythropoietin action. Eur J Biochem. 1992;210(3):649–663. - PubMed
-
- Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248(4953):378–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

