The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803
- PMID: 22086423
- PMCID: PMC3252115
- DOI: 10.1104/pp.111.184184
The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803
Abstract
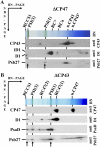
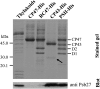
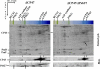
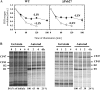
We have investigated the location of the Psb27 protein and its role in photosystem (PS) II biogenesis in the cyanobacterium Synechocystis sp. PCC 6803. Native gel electrophoresis revealed that Psb27 was present mainly in monomeric PSII core complexes but also in smaller amounts in dimeric PSII core complexes, in large PSII supercomplexes, and in the unassembled protein fraction. We conclude from analysis of assembly mutants and isolated histidine-tagged PSII subcomplexes that Psb27 associates with the "unassembled" CP43 complex, as well as with larger complexes containing CP43, possibly in the vicinity of the large lumenal loop connecting transmembrane helices 5 and 6 of CP43. A functional role for Psb27 in the biogenesis of CP43 is supported by the decreased accumulation and enhanced fragmentation of unassembled CP43 after inactivation of the psb27 gene in a mutant lacking CP47. Unexpectedly, in strains unable to assemble PSII, a small amount of Psb27 comigrated with monomeric and trimeric PSI complexes upon native gel electrophoresis, and Psb27 could be copurified with histidine-tagged PSI isolated from the wild type. Yeast two-hybrid assays suggested an interaction of Psb27 with the PsaB protein of PSI. Pull-down experiments also supported an interaction between CP43 and PSI. Deletion of psb27 did not have drastic effects on PSII assembly and repair but did compromise short-term acclimation to high light. The tentative interaction of Psb27 and CP43 with PSI raises the possibility that PSI might play a previously unrecognized role in the biogenesis/repair of PSII.
Figures



Similar articles
-
Association of Psb28 and Psb27 Proteins with PSII-PSI Supercomplexes upon Exposure of Synechocystis sp. PCC 6803 to High Light.Mol Plant. 2017 Jan 9;10(1):62-72. doi: 10.1016/j.molp.2016.08.001. Epub 2016 Aug 12. Mol Plant. 2017. PMID: 27530366
-
Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates.Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18536-41. doi: 10.1073/pnas.1111597108. Epub 2011 Oct 26. Proc Natl Acad Sci U S A. 2011. PMID: 22031695 Free PMC article.
-
Role of the PsbI protein in photosystem II assembly and repair in the cyanobacterium Synechocystis sp. PCC 6803.Plant Physiol. 2007 Dec;145(4):1681-91. doi: 10.1104/pp.107.107805. Epub 2007 Oct 5. Plant Physiol. 2007. PMID: 17921338 Free PMC article.
-
Structure and function of the hydrophilic Photosystem II assembly proteins: Psb27, Psb28 and Ycf48.Plant Physiol Biochem. 2014 Aug;81:96-107. doi: 10.1016/j.plaphy.2014.02.013. Epub 2014 Feb 25. Plant Physiol Biochem. 2014. PMID: 24656878 Review.
-
CP43-like chlorophyll binding proteins: structural and evolutionary implications.Trends Plant Sci. 2006 Mar;11(3):152-8. doi: 10.1016/j.tplants.2006.01.007. Epub 2006 Feb 13. Trends Plant Sci. 2006. PMID: 16473546 Review.
Cited by
-
Inhibition of chlorophyll biosynthesis at the protochlorophyllide reduction step results in the parallel depletion of Photosystem I and Photosystem II in the cyanobacterium Synechocystis PCC 6803.Planta. 2013 Feb;237(2):497-508. doi: 10.1007/s00425-012-1761-4. Epub 2012 Sep 26. Planta. 2013. PMID: 23011568
-
The Photosystem II Assembly Factor Ycf48 from the Cyanobacterium Synechocystis sp. PCC 6803 Is Lipidated Using an Atypical Lipobox Sequence.Int J Mol Sci. 2021 Apr 2;22(7):3733. doi: 10.3390/ijms22073733. Int J Mol Sci. 2021. PMID: 33918522 Free PMC article.
-
Psb34 protein modulates binding of high-light-inducible proteins to CP47-containing photosystem II assembly intermediates in the cyanobacterium Synechocystis sp. PCC 6803.Photosynth Res. 2022 Jun;152(3):333-346. doi: 10.1007/s11120-022-00908-9. Epub 2022 Mar 13. Photosynth Res. 2022. PMID: 35279779 Free PMC article.
-
Synergic Effects of Temperature and Irradiance on the Physiology of the Marine Synechococcus Strain WH7803.Front Microbiol. 2020 Jul 24;11:1707. doi: 10.3389/fmicb.2020.01707. eCollection 2020. Front Microbiol. 2020. PMID: 32793165 Free PMC article.
-
Regulatory factors for the assembly of thylakoid membrane protein complexes.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 19;367(1608):3420-9. doi: 10.1098/rstb.2012.0065. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23148269 Free PMC article. Review.
References
-
- Barber J. (2006) Photosystem II: an enzyme of global significance. Biochem Soc Trans 34: 619–631 - PubMed
-
- Bibby TS, Nield J, Barber J. (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412: 743–745 - PubMed
-
- Boekema EJ, Hifney A, Yakushevska AE, Piotrowski M, Keegstra W, Berry S, Michel KP, Pistorius EK, Kruip J. (2001) A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature 412: 745–748 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

