Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells
- PMID: 22086878
- PMCID: PMC3256094
- DOI: 10.1161/CIRCULATIONAHA.111.042598
Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells
Abstract
Background: Cardiosphere-derived cells (CDCs) are an attractive cell type for tissue regeneration, and autologous CDCs are being tested clinically. However, autologous therapy necessitates patient-specific tissue harvesting and cell processing, with delays to therapy and possible variations in cell potency. The use of allogeneic CDCs, if safe and effective, would obviate such limitations. We compared syngeneic and allogeneic CDC transplantation in rats from immunologically-mismatched inbred strains.
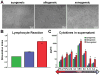
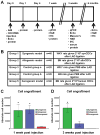
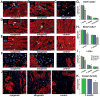
Methods and results: In vitro, CDCs expressed major histocompatibility complex class I but not class II antigens or B7 costimulatory molecules. In mixed-lymphocyte cocultures, allogeneic CDCs elicited negligible lymphocyte proliferation and inflammatory cytokine secretion. In vivo, syngeneic and allogeneic CDCs survived at similar levels in the infarcted rat heart 1 week after delivery, but few syngeneic (and even fewer allogeneic) CDCs remained at 3 weeks. Allogeneic CDCs induced a transient, mild, local immune reaction in the heart, without histologically evident rejection or systemic immunogenicity. Improvements in cardiac structure and function, sustained for 6 months, were comparable with syngeneic and allogeneic CDCs. Allogeneic CDCs stimulated endogenous regenerative mechanisms (cardiomyocyte cycling, recruitment of c-kit(+) cells, angiogenesis) and increased myocardial vascular endothelial growth factor, insulin-like growth factor-1, and hepatocyte growth factor equally with syngeneic CDCs.
Conclusions: Allogeneic CDC transplantation without immunosuppression is safe, promotes cardiac regeneration, and improves heart function in a rat myocardial infarction model, mainly through stimulation of endogenous repair mechanisms. The indirect mechanism of action rationalizes the persistence of benefit despite the evanescence of transplanted cell survival. This work motivates the testing of allogeneic human CDCs as a potential off-the-shelf product for cellular cardiomyoplasty.
Conflict of interest statement
Figures





References
-
- Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. - PubMed
-
- CADUCEUS. CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (NCT00893360) Available at: www.clinicaltrials.gov. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

