Acute glucocorticoid administration rapidly suppresses basal and stress-induced hypothalamo-pituitary-adrenal axis activity
- PMID: 22087024
- PMCID: PMC3279736
- DOI: 10.1210/en.2011-1434
Acute glucocorticoid administration rapidly suppresses basal and stress-induced hypothalamo-pituitary-adrenal axis activity
Abstract
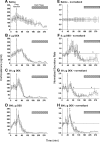
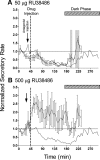
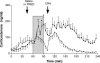
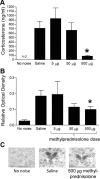
Hypothalamo-pituitary-adrenal (HPA) axis activity is subject to negative feedback control by glucocorticoids. Although the rapid component of this feedback is widely considered to contribute to regulation of dynamic HPA activity, few in vivo data exist on the temporal and pharmacological characteristics of this phenomenon. Thus, frequent automated blood sampling was undertaken in rats to determine the effects of acute glucocorticoid administration on basal and stress-induced corticosterone secretion. The glucocorticoid agonist methylprednisolone (5-2000 μg) or dexamethasone (5-500 μg) injected iv at the peak of the diurnal rhythm caused dose-dependent suppression of basal corticosterone secretion, which was attenuated by the glucocorticoid receptor antagonist RU38486. With 50 μg methylprednisolone, the onset of this suppression occurred at 40 min and remained significant for 120 min. However, although higher doses led to a greater and more sustained suppression of endogenous corticosterone, the response was delayed by the emergence of an initial stimulatory response that imposed a finite minimum delay. A corticosterone response to injection of CRH (1 μg, iv) during the period of maximal suppression indicated a suprapituitary site for the inhibitory effect glucocorticoid activation. This mechanism was supported by glucocorticoid injection immediately before a psychological stress (30 min, white noise); methylprednisolone caused dose-dependent attenuation of stress-induced corticosterone release and expression of the activity marker c-fos mRNA in the paraventricular nucleus but did not block the pituitary response to CRH. Thus, in rats, glucocorticoid receptor activation rapidly suppresses basal and stress-induced HPA activity that operates, at least in part, through a central mechanism of action.
Figures



References
-
- Jingami H, Matsukura S, Numa S, Imura H. 1985. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/β-lipotropin precursor mRNA in the pituitary in rats. Endocrinology 117:1314–1320 - PubMed
-
- Kovács KJ, Mezey E. 1987. Dexamethasone inhibits corticotropin-releasing factor gene expression in the rat paraventricular nucleus. Neuroendocrinology 46:365–368 - PubMed
-
- Drouin J, Sun YL, Nemer M. 1989. Glucocorticoid repression of pro-opiomelanocortin gene transcription. J Steroid Biochem 34:63–69 - PubMed
-
- Nakai Y, Usui T, Tsukada T, Takahashi H, Fukata J, Fukushima M, Senoo K, Imura H. 1991. Molecular mechanisms of glucocorticoid inhibition of human proopiomelanocortin gene transcription. J Steroid Biochem Mol Biol 40:301–306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

