Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells
- PMID: 22087232
- PMCID: PMC3210759
- DOI: 10.1371/journal.pone.0026580
Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells
Abstract
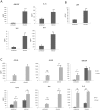
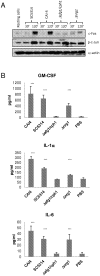
We previously reported that a bi-phasic innate immune MAPK response, constituting activation of the mitogen-activated protein kinase (MAPK) phosphatase MKP1 and c-Fos transcription factor, discriminates between the yeast and hyphal forms of Candida albicans in oral epithelial cells (ECs). Since the vast majority of mucosal Candida infections are vaginal, we sought to determine whether a similar bi-phasic MAPK-based immune response was activated by C. albicans in vaginal ECs. Here, we demonstrate that vaginal ECs orchestrate an innate response to C. albicans via NF-κB and MAPK signaling pathways. However, unlike in oral ECs, the first MAPK response, defined by c-Jun transcription factor activation, is delayed until 2 h in vaginal ECs but is still independent of hypha formation. The 'second' or 'late' MAPK response, constituting MKP1 and c-Fos transcription factor activation, is identical to oral ECs and is dependent upon both hypha formation and fungal burdens. NF-κB activation is immediate but independent of morphology. Furthermore, the proinflammatory response in vaginal ECs is different to oral ECs, with an absence of G-CSF and CCL20 and low level IL-6 production. Therefore, differences exist in how C. albicans activates signaling mechanisms in oral and vaginal ECs; however, the activation of MAPK-based pathways that discriminate between yeast and hyphal forms is retained between these mucosal sites. We conclude that this MAPK-based signaling pathway is a common mechanism enabling different human epithelial tissues to orchestrate innate immune responses specifically against C. albicans hyphae.
Conflict of interest statement
Figures




References
-
- Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Annals N Y Acad Sci. 1988;544:547–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

