Nutrient sensing kinases PKA and Sch9 phosphorylate the catalytic domain of the ubiquitin-conjugating enzyme Cdc34
- PMID: 22087249
- PMCID: PMC3210133
- DOI: 10.1371/journal.pone.0027099
Nutrient sensing kinases PKA and Sch9 phosphorylate the catalytic domain of the ubiquitin-conjugating enzyme Cdc34
Abstract
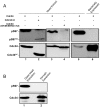
Cell division is controlled in part by the timely activation of the CDK, Cdc28, through its association with G1 and G2 cyclins. Cdc28 complexes are regulated in turn by the ubiquitin conjugating enzyme Cdc34 and SCF ubiquitin ligase complexes of the ubiquitin-proteasome system (UPS) to control the initiation of DNA replication. Here we demonstrate that the nutrient sensing kinases PKA and Sch9 phosphorylate S97 of Cdc34. S97 is conserved across species and restricted to the catalytic domain of Cdc34/Ubc7-like E2s. Cdc34-S97 phosphorylation is cell cycle regulated, elevated during active cell growth and division and decreased during cell cycle arrest. Cell growth and cell division are orchestrated to maintain cell size homeostasis over a wide range of nutrient conditions. Cells monitor changes in their environment through nutrient sensing protein kinases. Thus Cdc34 phosphorylation by PKA and Sch9 provides a direct tether between G1 cell division events and cell growth.
Conflict of interest statement
Figures








References
-
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
-
- Goebl MG, Yochem J, Jentsch S, McGrath JP, Varshavsky A, et al. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988;241:1331–1335. - PubMed
-
- Mathias N, Steussy CN, Goebl MG. An essential domain within Cdc34p is required for binding to a complex containing Cdc4p and Cdc53p in Saccharomyces cerevisiae. J Biol Chem. 1998;273:4040–4045. - PubMed
-
- Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

