Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms
- PMID: 22087285
- PMCID: PMC3210776
- DOI: 10.1371/journal.pone.0027306
Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms
Abstract
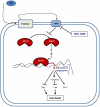
Background: Recent evidence from in vitro and in vivo studies has demonstrated that aberrant reactivation of the Sonic Hedgehog (SHH) signaling pathway regulates genes that promote cellular proliferation in various human cancer stem cells (CSCs). Therefore, the chemotherapeutic agents that inhibit activation of Gli transcription factors have emerged as promising novel therapeutic drugs for pancreatic cancer. GDC-0449 (Vismodegib), orally administrable molecule belonging to the 2-arylpyridine class, inhibits SHH signaling pathway by blocking the activities of Smoothened. The objectives of this study were to examine the molecular mechanisms by which GDC-0449 regulates human pancreatic CSC characteristics in vitro.
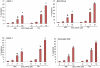
Methodology/principal findings: GDC-0499 inhibited cell viability and induced apoptosis in three pancreatic cancer cell lines and pancreatic CSCs. This inhibitor also suppressed cell viability, Gli-DNA binding and transcriptional activities, and induced apoptosis through caspase-3 activation and PARP cleavage in pancreatic CSCs. GDC-0449-induced apoptosis in CSCs showed increased Fas expression and decreased expression of PDGFRα. Furthermore, Bcl-2 was down-regulated whereas TRAIL-R1/DR4 and TRAIL-R2/DR5 expression was increased following the treatment of CSCs with GDC-0449. Suppression of both Gli1 plus Gli2 by shRNA mimicked the changes in cell viability, spheroid formation, apoptosis and gene expression observed in GDC-0449-treated pancreatic CSCs. Thus, activated Gli genes repress DRs and Fas expressions, up-regulate the expressions of Bcl-2 and PDGFRα and facilitate cell survival.
Conclusions/significance: These data suggest that GDC-0499 can be used for the management of pancreatic cancer by targeting pancreatic CSCs.
Conflict of interest statement
Figures





References
-
- Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26-29; discussion 29–30. - PubMed
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

