In Vitro Assessment of Apoptosis and Necrosis Following Cold Storage in a Human Airway Cell Model
- PMID: 22087352
- PMCID: PMC3205736
- DOI: 10.1089/bio.2009.0002
In Vitro Assessment of Apoptosis and Necrosis Following Cold Storage in a Human Airway Cell Model
Abstract
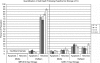
As advances in medical technology improve the efficacy of cell and tissue transplantation, a void remains in our knowledge base as to the specific molecular responses of cells to low-temperature storage. While much focus has been given to solution formulation for tissue perfusion during storage, investigations into cold exposure-induced complex molecular changes remain limited. The intent of this study was to quantify the levels of cell death following hypothermic storage in a lung cell model, establishing a foundation for future in-depth molecular analysis. Normal human lung fibroblasts (IMR-90) were stored for 1 day or 2 days and small airway epithelial cells (SAEC) were stored for 5 days or 7 days at 4°C in complete media, ViaSpan, or ViaSpan + pan-caspase (VI) inhibitor. (Poststorage viability was assessed for 3 days using alamarBlue(™).) Sample analysis revealed that IMR-90 cells stored in ViaSpan remained 80% (±9) viable after 1 day of storage and 21% (±7) viable after 2 days of storage. SAEC cells stored in ViaSpan remained 81% (±5) viable after 5 days and 28% (±7) after 7 days. Microfluidic flow cytometry analysis of the apoptotic and necrotic populations in the ViaSpan-stored samples revealed that in the IMR-90 cells stored for 2 days, 7% of the population was apoptotic at 4-h poststorage, while ∼70% was identified as necrotic. Analysis of the SAEC cell system following 7 days of ViaSpan storage revealed an apoptotic peak of 19% at 4-h poststorage and a corresponding necrotic peak of 19%. Caspase inhibition during hypothermic storage increased viability 33% for IMR-90 and 25% for SAEC. Data revealed a similar pattern of cell death, through both apoptosis and necrosis, once the onset of cold storage failure began, implying a potential conserved mechanism of cold-induced cell death. These data highlight the critical need for a more in-depth understanding of the molecular changes that occur as a result of cold exposure in cells and tissues.
Figures




References
-
- Toledo-Pereyra LH. Hau T. Simmons RL, et al. Lung preservation techniques. Ann Thorac Surg. 1977;23:487–494. - PubMed
-
- Lysaght MJ. Reyes J. The growth of tissue engineering. Tissue Eng. 2001;7:485–493. - PubMed
-
- Southard JH. Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. - PubMed
-
- Hicks M. Hing A. Gao L, et al. Organ preservation. Methods Mol Biol. 2006;333:331–374. - PubMed
-
- Baust JM. Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preserv Technol. 2002;1:17–31.
LinkOut - more resources
Full Text Sources
Research Materials

