C2'-pyrene-functionalized triazole-linked DNA: universal DNA/RNA hybridization probes
- PMID: 22087648
- PMCID: PMC3253902
- DOI: 10.1021/jo201845z
C2'-pyrene-functionalized triazole-linked DNA: universal DNA/RNA hybridization probes
Abstract
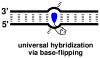
Development of universal hybridization probes, that is, oligonucleotides displaying identical affinity toward matched and mismatched DNA/RNA targets, has been a longstanding goal due to potential applications as degenerate PCR primers and microarray probes. The classic approach toward this end has been the use of "universal bases" that either are based on hydrogen-bonding purine derivatives or aromatic base analogues without hydrogen-bonding capabilities. However, development of probes that result in truly universal hybridization without compromising duplex thermostability has proven challenging. Here we have used the "click reaction" to synthesize four C2'-pyrene-functionalized triazole-linked 2'-deoxyuridine phosphoramidites. We demonstrate that oligodeoxyribonucleotides modified with the corresponding monomers display (a) minimally decreased thermal affinity toward DNA/RNA complements relative to reference strands, (b) highly robust universal hybridization characteristics (average differences in thermal denaturation temperatures of matched vs mismatched duplexes involving monomer W are <1.7 °C), and (c) exceptional affinity toward DNA targets containing abasic sites opposite of the modification site (ΔT(m) up to +25 °C). The latter observation, along with results from absorption and fluorescence spectroscopy, suggests that the pyrene moiety is intercalating into the duplex whereby the opposing nucleotide is pushed into an extrahelical position. These properties render C2'-pyrene-functionalized triazole-linked DNA as promising universal hybridization probes for applications in nucleic acid chemistry and biotechnology.
Figures



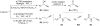
Similar articles
-
Recent Advances in Nucleic Acid Targeting Probes and Supramolecular Constructs Based on Pyrene-Modified Oligonucleotides.Molecules. 2017 Nov 30;22(12):2108. doi: 10.3390/molecules22122108. Molecules. 2017. PMID: 29189716 Free PMC article. Review.
-
Pyrene-functionalized triazole-linked 2'-deoxyuridines-probes for discrimination of single nucleotide polymorphisms (SNPs).Chem Commun (Camb). 2010 Jul 21;46(27):4929-31. doi: 10.1039/c0cc01133a. Epub 2010 Jun 4. Chem Commun (Camb). 2010. PMID: 20526503
-
Unlocked nucleic acids with a pyrene-modified uracil: synthesis, hybridization studies, fluorescent properties and i-motif stability.Chembiochem. 2014 Jan 3;15(1):146-56. doi: 10.1002/cbic.201300567. Chembiochem. 2014. PMID: 24501777
-
Recognition of Double-Stranded DNA Using Energetically Activated Duplexes Modified with N2'-Pyrene-, Perylene-, or Coronene-Functionalized 2'-N-Methyl-2'-amino-DNA Monomers.J Org Chem. 2015 Jun 5;80(11):5395-406. doi: 10.1021/acs.joc.5b00742. Epub 2015 May 22. J Org Chem. 2015. PMID: 25984765
-
DNA probes: applications of the principles of nucleic acid hybridization.Crit Rev Biochem Mol Biol. 1991;26(3-4):227-59. doi: 10.3109/10409239109114069. Crit Rev Biochem Mol Biol. 1991. PMID: 1718662 Review.
Cited by
-
Aptamers Chemistry: Chemical Modifications and Conjugation Strategies.Molecules. 2019 Dec 18;25(1):3. doi: 10.3390/molecules25010003. Molecules. 2019. PMID: 31861277 Free PMC article. Review.
-
Chemical architecture and applications of nucleic acid derivatives containing 1,2,3-triazole functionalities synthesized via click chemistry.Molecules. 2012 Oct 26;17(11):12665-703. doi: 10.3390/molecules171112665. Molecules. 2012. PMID: 23103533 Free PMC article. Review.
-
Two novel colorimetric fluorescent probes: Hg2+ and Al3+ in the visual colorimetric recognition environment.RSC Adv. 2020 Jan 16;10(6):3048-3059. doi: 10.1039/c9ra08428b. eCollection 2020 Jan 16. RSC Adv. 2020. PMID: 35497715 Free PMC article.
-
Conformational Control of Dual Emission by Pyrrolidinyl PNA-DNA Hybrids.ChemistryOpen. 2012 Aug;1(4):173-6. doi: 10.1002/open.201200016. Epub 2012 Jul 11. ChemistryOpen. 2012. PMID: 24551507 Free PMC article. No abstract available.
-
Recent Advances in Nucleic Acid Targeting Probes and Supramolecular Constructs Based on Pyrene-Modified Oligonucleotides.Molecules. 2017 Nov 30;22(12):2108. doi: 10.3390/molecules22122108. Molecules. 2017. PMID: 29189716 Free PMC article. Review.
References
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. - PubMed
-
- Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. - PubMed
-
- Ustinov AV, Stepanova IA, Dubnyakova VV, Zatsepin TS, Nozhevnikova EV, Korshun VA. Russ J Bioorg Chem. 2010;36:437–481. - PubMed
-
- El-Sagheer AH, Brown T. Chem Soc Rev. 2010;39:1388–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

