Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles
- PMID: 22088217
- PMCID: PMC3249476
- DOI: 10.1016/j.virol.2011.10.029
Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles
Abstract
Cholesterol and sphingolipid enriched lipid raft micro-domains in the plasma membrane play an important role in the life-cycle of numerous enveloped viruses. Although human respiratory syncytial virus (RSV) proteins associate with the raft domains of infected cells and rafts are incorporated in RSV virion particles, the functional role of raft during RSV infection was unknown. In the current study we have identified rafts as an essential component of host cell that is required for RSV infection. Treatment of human lung epithelial cells with raft disrupting agent methyl-beta-cyclodextrin (MBCD) led to drastic loss of RSV infectivity due to diminished release of infectious progeny RSV virion particles from raft disrupted cells. RSV infection of raft deficient Niemann-Pick syndrome type C human fibroblasts and normal human embryonic lung fibroblasts revealed that during productive RSV infection, raft is required for release of infectious RSV particles.
Copyright © 2011. Published by Elsevier Inc.
Figures
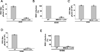
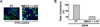
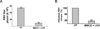
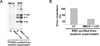


References
-
- Ali A, Nayak DP. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology. 2000;276:289–303. - PubMed
-
- Basu M, Maitra RK, Xiang Y, Meng X, Banerjee AK, Bose S. Inhibition of vesicular stomatitis virus infection in epithelial cells by alpha interferon-induced soluble secreted proteins. J. Gen. Virol. 2006;87:2653–2662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

