Pathophysiology of acute respiratory distress syndrome. Glucocorticoid receptor-mediated regulation of inflammation and response to prolonged glucocorticoid treatment
- PMID: 22088618
- PMCID: PMC9905212
- DOI: 10.1016/j.lpm.2011.04.023
Pathophysiology of acute respiratory distress syndrome. Glucocorticoid receptor-mediated regulation of inflammation and response to prolonged glucocorticoid treatment
Abstract
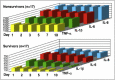
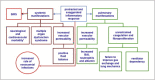
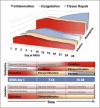
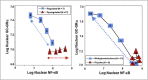
Based on molecular mechanisms and physiologic data, a strong association has been established between dysregulated systemic inflammation and progression of ARDS. In ARDS patients, glucocorticoid receptor-mediated down-regulation of systemic inflammation is essential to restore homeostasis, decrease morbidity and improve survival and can be significantly enhanced with prolonged low-to-moderate dose glucocorticoid treatment. A large body of evidence supports a strong association between prolonged glucocorticoid treatment-induced down-regulation of the inflammatory response and improvement in pulmonary and extrapulmonary physiology. The balance of the available data from controlled trials provides consistent strong level of evidence (grade 1B) for improving patient-centered outcomes. The sizable increase in mechanical ventilation-free days (weighted mean difference, 6.58 days; 95% CI, 2.93 -10.23; P<0.001) and ICU-free days (weighted mean difference, 7.02 days; 95% CI, 3.20-10.85; P<0.001) by day 28 is superior to any investigated intervention in ARDS. The largest meta-analysis on the subject concluded that treatment was associated with a significant risk reduction (RR=0.62, 95% CI: 0.43-0.91; P=0.01) in mortality and that the in-hospital number needed to treat to save one life was 4 (95% CI 2.4-10). The balance of the available data, however, originates from small controlled trials with a moderate degree of heterogeneity and provides weak evidence (grade 2B) for a survival benefit. Treatment decisions involve a tradeoff between benefits and risks, as well as costs. This low cost highly effective therapy is familiar to every physician and has a low risk profile when secondary prevention measures are implemented.
Copyright © 2011. Published by Elsevier Masson SAS.
Figures


Similar articles
-
Rationale for Prolonged Glucocorticoid Use in Pediatric ARDS: What the Adults Can Teach Us.Front Pediatr. 2016 Jun 14;4:58. doi: 10.3389/fped.2016.00058. eCollection 2016. Front Pediatr. 2016. PMID: 27379217 Free PMC article. Review.
-
Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy.Chest. 2009 Dec;136(6):1631-1643. doi: 10.1378/chest.08-2408. Epub 2009 Oct 3. Chest. 2009. PMID: 19801579 Review.
-
Glucocorticoid treatment in acute lung injury and acute respiratory distress syndrome.Crit Care Clin. 2011 Jul;27(3):589-607. doi: 10.1016/j.ccc.2011.05.007. Crit Care Clin. 2011. PMID: 21742218 Review.
-
Prolonged glucocorticoid treatment and secondary prevention in acute respiratory distress syndrome.Expert Rev Respir Med. 2010 Apr;4(2):201-10. doi: 10.1586/ers.10.2. Expert Rev Respir Med. 2010. PMID: 20406086 Review.
-
Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids.Neuroimmunomodulation. 2005;12(6):321-38. doi: 10.1159/000091126. Neuroimmunomodulation. 2005. PMID: 16557033 Clinical Trial.
Cited by
-
Dexamethasone ameliorates H₂S-induced acute lung injury by alleviating matrix metalloproteinase-2 and -9 expression.PLoS One. 2014 Apr 10;9(4):e94701. doi: 10.1371/journal.pone.0094701. eCollection 2014. PLoS One. 2014. PMID: 24722316 Free PMC article.
-
The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach.Life Sci. 2020 Oct 1;258:118166. doi: 10.1016/j.lfs.2020.118166. Epub 2020 Jul 31. Life Sci. 2020. PMID: 32739471 Free PMC article. Review.
-
Rationale for Prolonged Glucocorticoid Use in Pediatric ARDS: What the Adults Can Teach Us.Front Pediatr. 2016 Jun 14;4:58. doi: 10.3389/fped.2016.00058. eCollection 2016. Front Pediatr. 2016. PMID: 27379217 Free PMC article. Review.
-
The Treatment of Tubal Inflammatory Infertility using Yinjia Tablets through EGFR/MEK/ERK Signaling Pathway based on Network Pharmacology.Curr Pharm Biotechnol. 2024;25(4):499-509. doi: 10.2174/0113892010234591230919074245. Curr Pharm Biotechnol. 2024. PMID: 38572608
-
Preventing the development of severe COVID-19 by modifying immunothrombosis.Life Sci. 2021 Jan 1;264:118617. doi: 10.1016/j.lfs.2020.118617. Epub 2020 Oct 20. Life Sci. 2021. PMID: 33096114 Free PMC article. Review.
References
-
- Jantz M.A., Sahn S.A. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999;160(4):1079–1100. - PubMed
-
- Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. - PubMed
-
- Meduri G.U., Annane D., Chrousos G.P., Marik P.E., Sinclair S.E. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–1643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

