Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis
- PMID: 22089247
- PMCID: PMC3255948
- DOI: 10.1128/CVI.05414-11
Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis
Abstract
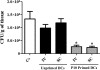
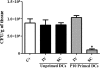
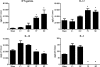
Vaccination with peptide 10 (P10), derived from the Paracoccidioides brasiliensis glycoprotein 43 (gp43), induces a Th1 response that protects mice in an intratracheal P. brasiliensis infection model. Combining P10 with complete Freund's adjuvant (CFA) or other adjuvants further increases the peptide's antifungal effect. Since dendritic cells (DCs) are up to 1,000-fold more efficient at activating T cells than CFA, we examined the impact of P10-primed bone-marrow-derived DC vaccination in mice. Splenocytes from mice immunized with P10 were stimulated in vitro with P10 or P10-primed DCs. T cell proliferation was significantly increased in the presence of P10-primed DCs compared to the peptide. The protective efficacy of P10-primed DCs was studied in an intratracheal P. brasiliensis model in BALB/c mice. Administration of P10-primed DCs prior to (via subcutaneous vaccination) or weeks after (via either subcutaneous or intravenous injection) P. brasiliensis infection decreased pulmonary damage and significantly reduced fungal burdens. The protective response mediated by the injection of primed DCs was characterized mainly by an increased production of gamma interferon (IFN-γ) and interleukin 12 (IL-12) and a reduction in IL-10 and IL-4 compared to those of infected mice that received saline or unprimed DCs. Hence, our data demonstrate the potential of P10-primed DCs as a vaccine capable of both the rapid protection against the development of serious paracoccidioidomycosis or the treatment of established P. brasiliensis disease.
Figures
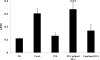



Similar articles
-
Immunization with P10 peptide increases specific immunity and protects immunosuppressed BALB/c mice infected with virulent yeasts of Paracoccidioides brasiliensis.Mycopathologia. 2014 Oct;178(3-4):177-88. doi: 10.1007/s11046-014-9801-1. Epub 2014 Aug 19. Mycopathologia. 2014. PMID: 25135302
-
Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice.Infect Immun. 1998 Feb;66(2):786-93. doi: 10.1128/IAI.66.2.786-793.1998. Infect Immun. 1998. PMID: 9453642 Free PMC article.
-
Paracoccidioides brasiliensis vaccine formulations based on the gp43-derived P10 sequence and the Salmonella enterica FliC flagellin.Infect Immun. 2009 Apr;77(4):1700-7. doi: 10.1128/IAI.01470-08. Epub 2009 Feb 9. Infect Immun. 2009. PMID: 19204092 Free PMC article.
-
Attempts at a peptide vaccine against paracoccidioidomycosis, adjuvant to chemotherapy.Mycopathologia. 2008 Apr-May;165(4-5):341-52. doi: 10.1007/s11046-007-9056-1. Mycopathologia. 2008. PMID: 18777638 Review.
-
Paracoccidioidomycosis vaccine.Hum Vaccin Immunother. 2012 Oct;8(10):1450-3. doi: 10.4161/hv.21283. Epub 2012 Aug 16. Hum Vaccin Immunother. 2012. PMID: 22894948 Free PMC article. Review.
Cited by
-
Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics.J Fungi (Basel). 2023 May 31;9(6):633. doi: 10.3390/jof9060633. J Fungi (Basel). 2023. PMID: 37367569 Free PMC article. Review.
-
New advances in the development of a vaccine against paracoccidioidomycosis.Front Microbiol. 2012 Jun 12;3:212. doi: 10.3389/fmicb.2012.00212. eCollection 2012. Front Microbiol. 2012. PMID: 22701452 Free PMC article.
-
Dendritic cell interactions with Histoplasma and Paracoccidioides.Virulence. 2015;6(5):424-32. doi: 10.4161/21505594.2014.965586. Epub 2015 May 1. Virulence. 2015. PMID: 25933034 Free PMC article. Review.
-
Experimental Therapy of Paracoccidioidomycosis Using P10-Primed Monocyte-Derived Dendritic Cells Isolated From Infected Mice.Front Microbiol. 2019 Jul 31;10:1727. doi: 10.3389/fmicb.2019.01727. eCollection 2019. Front Microbiol. 2019. PMID: 31417520 Free PMC article.
-
Paracoccidioidomycosis Protective Immunity.J Fungi (Basel). 2021 Feb 13;7(2):137. doi: 10.3390/jof7020137. J Fungi (Basel). 2021. PMID: 33668671 Free PMC article. Review.
References
-
- Almeida SR, Lopes JD. 2001. The low efficiency of dendritic cells and macrophages from mice susceptible to Paracoccidioides brasiliensis in inducing a Th1 response. Braz. J. Med. Biol. Res. 34: 529–537 - PubMed
-
- Austyn JM. 2000. Antigen-presenting cells. Experimental and clinical studies of dendritic cells. Am. J. Respir. Crit. Care Med. 162 (Part 2): S146–S150 - PubMed
-
- Banchereau J, et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18: 767–811 - PubMed
-
- Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

