R7BP modulates opiate analgesia and tolerance but not withdrawal
- PMID: 22089315
- PMCID: PMC3280654
- DOI: 10.1038/npp.2011.284
R7BP modulates opiate analgesia and tolerance but not withdrawal
Abstract
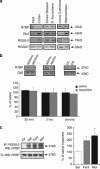
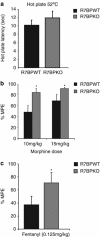
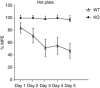
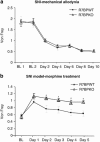
The adaptor protein R7 family binding protein (R7BP) modulates G protein coupled receptor (GPCR) signaling and desensitization by controlling the function of regulator of G protein signaling (RGS) proteins. R7BP is expressed throughout the brain and appears to modulate the membrane localization and stability of three proteins that belong to R7 RGS family: RGS6, RGS7, and RGS9-2. RGS9-2 is a potent negative modulator of opiate and psychostimulant addiction and promotes the development of analgesic tolerance to morphine, whereas the role of RGS6 and RGS7 in addiction remains unknown. Recent studies revealed that functional deletion of R7BP reduces R7 protein activity by preventing their anchoring to the cell membrane and enhances GPCR responsiveness in the basal ganglia. Here, we take advantage of R7BP knockout mice in order to examine the way interventions in R7 proteins function throughout the brain affect opiate actions. Our results suggest that R7BP is a negative modulator of the analgesic and locomotor activating actions of morphine. We also report that R7BP contributes to the development of morphine tolerance. Finally, our data suggest that although prevention of R7BP actions enhances the analgesic responses to morphine, it does not affect the severity of somatic withdrawal signs. Our data suggest that interventions in R7BP actions enhance the analgesic effect of morphine and prevent tolerance, without affecting withdrawal, pointing to R7BP complexes as potential new targets for analgesic drugs.
Figures

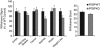
Similar articles
-
Nucleus accumbens-specific interventions in RGS9-2 activity modulate responses to morphine.Neuropsychopharmacology. 2014 Jul;39(8):1968-77. doi: 10.1038/npp.2014.45. Epub 2014 Feb 24. Neuropsychopharmacology. 2014. PMID: 24561386 Free PMC article.
-
Association with the Plasma Membrane Is Sufficient for Potentiating Catalytic Activity of Regulators of G Protein Signaling (RGS) Proteins of the R7 Subfamily.J Biol Chem. 2016 Mar 25;291(13):7195-204. doi: 10.1074/jbc.M115.713446. Epub 2016 Jan 25. J Biol Chem. 2016. PMID: 26811338 Free PMC article.
-
Regulator of G-Protein Signaling 7 Regulates Reward Behavior by Controlling Opioid Signaling in the Striatum.Biol Psychiatry. 2016 Aug 1;80(3):235-45. doi: 10.1016/j.biopsych.2015.07.026. Epub 2015 Aug 14. Biol Psychiatry. 2016. PMID: 26364547 Free PMC article.
-
R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling.Trends Pharmacol Sci. 2009 Jan;30(1):17-24. doi: 10.1016/j.tips.2008.10.002. Epub 2008 Nov 29. Trends Pharmacol Sci. 2009. PMID: 19042037 Free PMC article. Review.
-
Do pharmacological approaches that prevent opioid tolerance target different elements in the same regulatory machinery?Curr Drug Abuse Rev. 2008 Jun;1(2):222-38. doi: 10.2174/1874473710801020222. Curr Drug Abuse Rev. 2008. PMID: 19630721 Review.
Cited by
-
Orphan Receptor GPR158 Is an Allosteric Modulator of RGS7 Catalytic Activity with an Essential Role in Dictating Its Expression and Localization in the Brain.J Biol Chem. 2015 May 29;290(22):13622-39. doi: 10.1074/jbc.M115.645374. Epub 2015 Mar 19. J Biol Chem. 2015. PMID: 25792749 Free PMC article.
-
RGS9-2 Modulates Responses to Oxycodone in Pain-Free and Chronic Pain States.Neuropsychopharmacology. 2017 Jun;42(7):1548-1556. doi: 10.1038/npp.2017.4. Epub 2017 Jan 11. Neuropsychopharmacology. 2017. PMID: 28074831 Free PMC article.
-
Ptchd1 mediates opioid tolerance via cholesterol-dependent effects on μ-opioid receptor trafficking.Nat Neurosci. 2022 Sep;25(9):1179-1190. doi: 10.1038/s41593-022-01135-0. Epub 2022 Aug 18. Nat Neurosci. 2022. PMID: 35982154 Free PMC article.
-
RGS proteins as targets in the treatment of intestinal inflammation and visceral pain: New insights and future perspectives.Bioessays. 2016 Apr;38(4):344-54. doi: 10.1002/bies.201500118. Epub 2016 Jan 28. Bioessays. 2016. PMID: 26817719 Free PMC article. Review.
-
RGS9-2 modulates sensory and mood related symptoms of neuropathic pain.Neurobiol Learn Mem. 2014 Nov;115:43-8. doi: 10.1016/j.nlm.2014.08.005. Epub 2014 Aug 19. Neurobiol Learn Mem. 2014. PMID: 25150149 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

