Xpr1 is an atypical G-protein-coupled receptor that mediates xenotropic and polytropic murine retrovirus neurotoxicity
- PMID: 22090134
- PMCID: PMC3264382
- DOI: 10.1128/JVI.06073-11
Xpr1 is an atypical G-protein-coupled receptor that mediates xenotropic and polytropic murine retrovirus neurotoxicity
Abstract
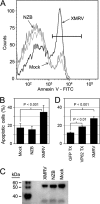
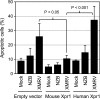
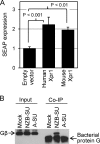
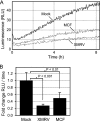
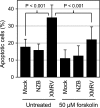
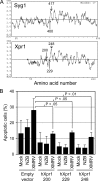
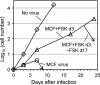
Xenotropic murine leukemia virus-related virus (XMRV) was first identified in human prostate cancer tissue and was later found in a high percentage of humans with chronic fatigue syndrome (CFS). While exploring potential disease mechanisms, we found that XMRV infection induced apoptosis in SY5Y human neuroblastoma cells, suggesting a mechanism for the neuromuscular pathology seen in CFS. Several lines of evidence show that the cell entry receptor for XMRV, Xpr1, mediates this effect, and chemical cross-linking studies show that Xpr1 is associated with the Gβ subunit of the G-protein heterotrimer. The activation of adenylate cyclase rescued the cells from XMRV toxicity, indicating that toxicity resulted from reduced G-protein-mediated cyclic AMP (cAMP) signaling. Some proteins with similarity to Xpr1 are involved in phosphate uptake into cells, but we found no role of Xpr1 in phosphate uptake or its regulation. Our results indicate that Xpr1 is a novel, atypical G-protein-coupled receptor (GPCR) and that xenotropic or polytropic retrovirus binding can disrupt the cAMP-mediated signaling function of Xpr1, leading to the apoptosis of infected cells. We show that this pathway is also responsible for the classic toxicity of the polytropic mink cell focus-forming (MCF) retrovirus in mink cells. Although it now seems clear that the detection of XMRV in humans was the result of sample contamination with a recombinant mouse virus, our findings may have relevance to neurologic disease induced by MCF retroviruses in mice.
Figures

References
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. 1978. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 38: 3751–3757 - PubMed
-
- Bigner DD, et al. 1981. Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J. Neuropathol. Exp. Neurol. 40: 201–229 - PubMed
-
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

