Codelivery of the chemokine CCL3 by an adenovirus-based vaccine improves protection from retrovirus infection
- PMID: 22090142
- PMCID: PMC3264363
- DOI: 10.1128/JVI.06244-11
Codelivery of the chemokine CCL3 by an adenovirus-based vaccine improves protection from retrovirus infection
Abstract
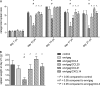
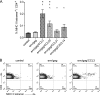
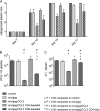
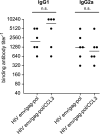
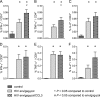
Processing and presentation of vaccine antigens by professional antigen-presenting cells (APCs) is of great importance for the efficient induction of protective immunity. We analyzed whether the efficacy of an adenovirus-based retroviral vaccine can be enhanced by coadministration of adenovirus-encoded chemokines that attract and stimulate APCs. In the Friend retrovirus (FV) mouse model we coexpressed CCL3, CCL20, CCL21, or CXCL14 from adenoviral vectors, together with FV Gag and Env antigens, and then analyzed immune responses and protection from pathogenic FV infection. Although most tested chemokines did not improve protection against FV challenge, mice that received adenoviral vectors encoding CCL3 together with FV antigens showed significantly better control over viral loads and FV-induced disease than mice immunized with the viral antigens only. Improved protection correlated with enhanced virus-specific CD4+ T cell responses and higher neutralizing antibody titers. To apply these results to an HIV vaccine, mice were immunized with adenoviral vectors encoding the HIV antigens Env and Gag-Pol and coadministered vectors encoding CCL3. Again, this combination vaccine induced higher virus-specific antibody titers and CD4+ T cell responses than did the HIV antigens alone. These results indicate that coexpression of the chemokine CCL3 by adenovirus-based vectors may be a promising tool to improve antiretroviral vaccination strategies.
Figures
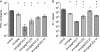

References
-
- Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252 - PubMed
-
- Bayer W, Schimmer S, Hoffmann D, Dittmer U, Wildner O. 2008. Evaluation of the Friend virus model for the development of improved adenovirus-vectored anti-retroviral vaccination strategies. Vaccine 26: 716–726 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

