Comparison of the hybrid capture 2 and cobas 4800 tests for detection of high-risk human papillomavirus in specimens collected in PreservCyt medium
- PMID: 22090403
- PMCID: PMC3256689
- DOI: 10.1128/JCM.05400-11
Comparison of the hybrid capture 2 and cobas 4800 tests for detection of high-risk human papillomavirus in specimens collected in PreservCyt medium
Abstract
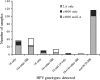
Clinical cervical cytology specimens (n = 466) collected in PreservCyt (Hologic Inc.) were used to evaluate the agreement between Hybrid Capture 2 (hc2; Qiagen) and cobas 4800 (c4800; Roche Molecular Diagnostics) for the detection of high-risk human papillomavirus (HR HPV) genotype infections. The agreement between the two assays was 93.8% (kappa = 0.87; 95% confidence interval, 0.828 to 0.918), with 186 and 251 concordant positive and negative results, respectively. All 186 concordant positives were confirmed using the Linear Array (LA; Roche Molecular Diagnostics) genotyping test. Of the 29 samples with discordant results (6.2%), 18 were hc2 positive and LA verified 17 as positive for HR HPV. Eleven discordant specimens were c4800 positive, and LA confirmed 5 as positive for HR HPV. As of October 2009, practice guidelines in Alberta, Canada, recommend reflex HPV testing for women over 30 years old with atypical squamous cells of undetermined significance (ASCUS) and for women over 50 years old with low-grade squamous intraepithelial lesions (LSIL) to help prioritize those who should undergo further evaluation. In this study, agreement between hc2 and c4800 results for samples from women over 30 years old with ASCUS cytology was 92.3% (n = 13), while no samples from women over 50 years old with LSIL cytology were identified for analysis.
Figures
References
-
- Bachtiary B, et al. 2002. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int. J. Cancer 102:237–243 - PubMed
-
- Bosch FX, de Sanjosé S. 2003. Chapter 1: human papillomavirus and cervical cancer—burden and assessment of causality. J. Natl. Cancer Inst. Monogr. 31:3–13 - PubMed
-
- Castle PE, et al. 2002. Restricted cross-reactivity of Hybrid Capture 2 with nononcogenic human papillomavirus types. Cancer Epidemiol. Biomarkers Prev. 11:1394–1399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials