Disrupting effect of drug-induced reward on spatial but not cue-guided learning: implication of the striatal protein kinase A/cAMP response element-binding protein pathway
- PMID: 22090478
- PMCID: PMC6633299
- DOI: 10.1523/JNEUROSCI.1787-11.2011
Disrupting effect of drug-induced reward on spatial but not cue-guided learning: implication of the striatal protein kinase A/cAMP response element-binding protein pathway
Abstract
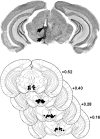
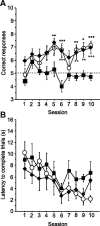
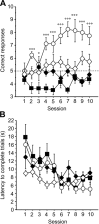
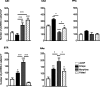
The multiple memory systems hypothesis posits that different neural circuits function in parallel and may compete for information processing and storage. For example, instrumental conditioning would depend on the striatum, whereas spatial memory may be mediated by a circuit centered on the hippocampus. However, the nature of the task itself is not sufficient to select durably one system over the other. In this study, we investigated the effects of natural and pharmacological rewards on the selection of a particular memory system during learning. We compared the effects of food- or drug-induced activation of the reward system on cue-guided versus spatial learning using a Y-maze discrimination task. Drug-induced reward severely impaired the acquisition of a spatial discrimination task but spared the cued version of the task. Immunohistochemical analysis of the phosphorylated form of the cAMP response element binding (CREB) protein and c-Fos expression induced by behavioral testing revealed that the spatial deficit was associated with a decrease of both markers within the hippocampus and the prefrontal cortex. In contrast, drug reward potentiated the cued learning-induced CREB phosphorylation within the dorsal striatum. Administration of the protein kinase A inhibitor 8-Bromo-adenosine-3',5'-cyclic monophosphorothioate Rp isomer (Rp-cAMPS) into the dorsal striatum before training completely reversed the drug-induced spatial deficit and restored CREB phosphorylation levels within the hippocampus and the prefrontal cortex. Therefore, drug-induced striatal hyperactivity may underlie the declarative memory deficit reported here. This mechanism could represent an important early step toward the development of addictive behaviors by promoting conditioning to the detriment of more flexible forms of memory.
Figures

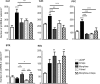
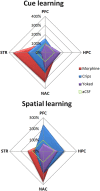
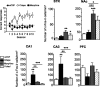
Similar articles
-
HDAC inhibition facilitates the switch between memory systems in young but not aged mice.J Neurosci. 2013 Jan 30;33(5):1954-63. doi: 10.1523/JNEUROSCI.3453-12.2013. J Neurosci. 2013. PMID: 23365234 Free PMC article.
-
Morphine Reward Promotes Cue-Sensitive Learning: Implication of Dorsal Striatal CREB Activity.Front Psychiatry. 2017 May 30;8:87. doi: 10.3389/fpsyt.2017.00087. eCollection 2017. Front Psychiatry. 2017. PMID: 28611691 Free PMC article.
-
Cyclic AMP and mitogen-activated protein kinases are required for glutamate-dependent cyclic AMP response element binding protein and Elk-1 phosphorylation in the dorsal striatum in vivo.J Neurochem. 2001 Jan;76(2):401-12. doi: 10.1046/j.1471-4159.2001.00051.x. J Neurochem. 2001. PMID: 11208903
-
Protein kinases in natural versus drug reward.Pharmacol Biochem Behav. 2022 Nov;221:173472. doi: 10.1016/j.pbb.2022.173472. Epub 2022 Oct 13. Pharmacol Biochem Behav. 2022. PMID: 36244528 Review.
-
Protein kinases and addiction.Ann N Y Acad Sci. 2008 Oct;1141:22-57. doi: 10.1196/annals.1441.022. Ann N Y Acad Sci. 2008. PMID: 18991950 Free PMC article. Review.
Cited by
-
Postsynaptic adenosine A2A receptors modulate intrinsic excitability of pyramidal cells in the rat basolateral amygdala.Int J Neuropsychopharmacol. 2015 Feb 25;18(6):pyv017. doi: 10.1093/ijnp/pyv017. Int J Neuropsychopharmacol. 2015. PMID: 25716780 Free PMC article.
-
Chronic alcohol consumption shifts learning strategies and synaptic plasticity from hippocampus to striatum-dependent pathways.Front Psychiatry. 2023 May 26;14:1129030. doi: 10.3389/fpsyt.2023.1129030. eCollection 2023. Front Psychiatry. 2023. PMID: 37304443 Free PMC article.
-
Differences in the Flexibility of Switching Learning Strategies and CREB Phosphorylation Levels in Prefrontal Cortex, Dorsal Striatum and Hippocampus in Two Inbred Strains of Mice.Front Behav Neurosci. 2016 Sep 16;10:176. doi: 10.3389/fnbeh.2016.00176. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27695401 Free PMC article.
-
The Potential Role of PKA/CREB Signaling Pathway Concerned with Gastrodin Administration on Methamphetamine-Induced Conditioned Place Preference Rats and SH-SY5Y Cell Line.Neurotox Res. 2020 Apr;37(4):926-935. doi: 10.1007/s12640-019-00150-7. Epub 2020 Jan 4. Neurotox Res. 2020. PMID: 31900897
-
Heads for learning, tails for memory: reward, reinforcement and a role of dopamine in determining behavioral relevance across multiple timescales.Front Neurosci. 2013 Oct 11;7:175. doi: 10.3389/fnins.2013.00175. Front Neurosci. 2013. PMID: 24130514 Free PMC article. Review.
References
-
- Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–128. - PubMed
-
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. - PubMed
-
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
