Use dependence of presynaptic tenacity
- PMID: 22090503
- PMCID: PMC6633291
- DOI: 10.1523/JNEUROSCI.3384-11.2011
Use dependence of presynaptic tenacity
Abstract
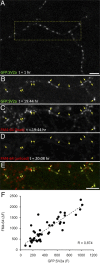
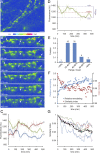
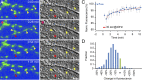
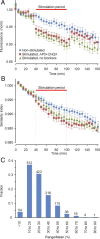
Recent studies indicate that synaptic vesicles (SVs) are continuously interchanged among nearby synapses at very significant rates. These dynamics and the lack of obvious barriers confining synaptic vesicles to specific synapses would seem to challenge the ability of synapses to maintain a constant amount of synaptic vesicles over prolonged time scales. Moreover, the extensive mobilization of synaptic vesicles associated with presynaptic activity might be expected to intensify this challenge. Here we examined the ability of individual presynaptic boutons of rat hippocampal neurons to maintain their synaptic vesicle content, and the degree to which this ability is affected by continuous activity. We found that the synaptic vesicle content of individual boutons belonging to the same axons gradually changed over several hours, and that these changes occurred independently of activity. Intermittent stimulation for 1 h accelerated rates of vesicle pool size change. Interestingly, however, following stimulation cessation, vesicle pool size change rates gradually converged with basal change rates. Over similar time scales, active zones (AZs) exhibited substantial remodeling; yet, unlike synaptic vesicles, AZ remodeling was not affected by the stimulation paradigms used here. These findings indicate that enhanced activity levels can increase synaptic vesicle redistribution among nearby synapses, but also highlight the presence of forces that act to restore particular set points in terms of SV contents, and support a role for active zones in preserving such set points. These findings also indicate, however, that neither AZ size nor SV content set points are particularly stable, questioning the long-term tenacity of presynaptic specializations.
Figures



References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. - PubMed
-
- Brigidi GS, Bamji SX. Cadherin-catenin adhesion complexes at the synapse. Curr Opin Neurobiol. 2011;21:208–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
