Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial
- PMID: 22093187
- PMCID: PMC3671878
- DOI: 10.1016/S0140-6736(11)61226-9
Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial
Abstract
Background: Bone metastases are a major cause of morbidity and mortality in men with prostate cancer. Preclinical studies suggest that osteoclast inhibition might prevent bone metastases. We assessed denosumab, a fully human anti-RANKL monoclonal antibody, for prevention of bone metastasis or death in non-metastatic castration-resistant prostate cancer.
Methods: In this phase 3, double-blind, randomised, placebo-controlled study, men with non-metastatic castration-resistant prostate cancer at high risk of bone metastasis (prostate-specific antigen [PSA] ≥8·0 μg/L or PSA doubling time ≤10·0 months, or both) were enrolled at 319 centres from 30 countries. Patients were randomly assigned (1:1) via an interactive voice response system to receive subcutaneous denosumab 120 mg or subcutaneous placebo every 4 weeks. Randomisation was stratified by PSA eligibility criteria and previous or ongoing chemotherapy for prostate cancer. Patients, investigators, and all people involved in study conduct were masked to treatment allocation. The primary endpoint was bone-metastasis-free survival, a composite endpoint determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause. Efficacy analysis was by intention to treat. The masked treatment phase of the trial has been completed. This trial was registered at ClinicalTrials.gov, number NCT00286091.
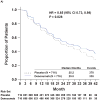
Findings: 1432 patients were randomly assigned to treatment groups (716 denosumab, 716 placebo). Denosumab significantly increased bone-metastasis-free survival by a median of 4·2 months compared with placebo (median 29·5 [95% CI 25·4-33·3] vs 25·2 [22·2-29·5] months; hazard ratio [HR] 0·85, 95% CI 0·73-0·98, p=0·028). Denosumab also significantly delayed time to first bone metastasis (33·2 [95% CI 29·5-38·0] vs 29·5 [22·4-33·1] months; HR 0·84, 95% CI 0·71-0·98, p=0·032). Overall survival did not differ between groups (denosumab, 43·9 [95% CI 40·1-not estimable] months vs placebo, 44·8 [40·1-not estimable] months; HR 1·01, 95% CI 0·85-1·20, p=0·91). Rates of adverse events and serious adverse events were similar in both groups, except for osteonecrosis of the jaw and hypocalcaemia. 33 (5%) patients on denosumab developed osteonecrosis of the jaw versus none on placebo. Hypocalcaemia occurred in 12 (2%) patients on denosumab and two (<1%) on placebo.
Interpretation: This large randomised study shows that targeting of the bone microenvironment can delay bone metastasis in men with prostate cancer.
Funding: Amgen Inc.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
M Smith, R Coleman, K Fizazi, and F Saad have been consultants for Amgen and Novartis; and L Karsh, B Tombal, H Van Poppel, J Chin, J Morote, K Miller, P Sieber, TL Tammela, and N Shore have been consultants for Amgen.
K Fizazi, F Saad, R Coleman, and B Tombal have participated in speakers’ bureaus for Amgen and Novartis. N Shore, K Miller, P Sieber, T Borkowski, and J Morote have participated in speakers’ bureaus for Amgen. K Fizazi has received travel funds from Amgen and Novartis; and B Egerdie, L Karsh, B Tombal, J Chin, K Miller, T Borkowski, and N Shore have received travel funds from Amgen. M Smith, F Saad, L Karsh, TL Tammela, and N Shore have received research funding from Amgen; and R Coleman has received research funding from Novartis. R Coleman has received honoraria from Amgen and Novartis; and M Smith, L Karsh, and N Shore have received honoraria from Amgen. R Coleman has provided expert testimony for Novartis. F Gomez Veiga and R Damião have no conflicts of interest to disclose. Z Ye, A Kupic, R Dansey, and C Goessl are employees of Amgen and have received stock/stock options from Amgen.
Figures




Comment in
-
Treatment of prostate cancer metastases: more than semantics.Lancet. 2012 Jan 7;379(9810):4-6. doi: 10.1016/S0140-6736(11)61540-7. Epub 2011 Nov 15. Lancet. 2012. PMID: 22093188 No abstract available.
-
Prostate cancer: does denosumab have a role in metastasis prevention?Nat Rev Urol. 2011 Dec 27;9(1):1. doi: 10.1038/nrurol.2011.196. Nat Rev Urol. 2011. PMID: 22200817 No abstract available.
-
Re: Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial.J Urol. 2012 Jun;187(6):2098. doi: 10.1016/j.juro.2012.03.041. Epub 2012 Apr 12. J Urol. 2012. PMID: 22579177 No abstract available.
-
Denosumab in castration-resistant prostate cancer.Lancet. 2012 May 12;379(9828):e50; author reply e50-1. doi: 10.1016/S0140-6736(12)60764-8. Lancet. 2012. PMID: 22579323 No abstract available.
-
Commentary on "denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial." M.R. Smith, F. Saad, R. Coleman, N. Shore, K. Fizazi, B. Tombal, K. Miller, P. Sieber, L. Karsh, R. Damião, T.L. Tammela, B. Egerdie, H. Van Poppel, J. Chin, J. Morote, F. Gómez-Veiga, T. Borkowski, Z. Ye, A. Kupic, R. Dansey, C. Goessl, Massachusetts General Hospital Cancer Center, Boston, MA 02114, USA.: Lancet 2012;379:39-46 [Epub;2011, November 15].Urol Oncol. 2012 Sep;30(5):747. doi: 10.1016/j.urolonc.2012.06.008. Urol Oncol. 2012. PMID: 23021561 No abstract available.
References
-
- Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010 Jul;184(1):162–7. - PubMed
-
- Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Prostate Cancer and Prostatic Disease. 2011. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. [Epub ahead of print] - PubMed
-
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

