Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis
- PMID: 22094253
- PMCID: PMC3487108
- DOI: 10.1016/j.ccr.2011.09.009
Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis
Abstract
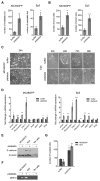
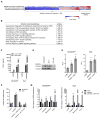
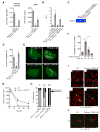
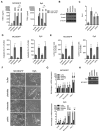
Interactions of cancer cells with the primary tumor microenvironment are important determinants of cancer progression toward metastasis but it is unknown whether additional prometastatic signals are provided during the intravascular transit to the site of metastasis. Here, we show that platelet-tumor cell interactions are sufficient to prime tumor cells for subsequent metastasis. Platelet-derived TGFβ and direct platelet-tumor cell contacts synergistically activate the TGFβ/Smad and NF-κB pathways in cancer cells, resulting in their transition to an invasive mesenchymal-like phenotype and enhanced metastasis in vivo. Inhibition of NF-κB signaling in cancer cells or ablation of TGFβ1 expression solely in platelets protects against lung metastasis in vivo. Thus, cancer cells rely on platelet-derived signals outside of the primary tumor for efficient metastasis.
2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Platelets alter tumor cell attributes to propel metastasis: programming in transit.Cancer Cell. 2011 Nov 15;20(5):553-4. doi: 10.1016/j.ccr.2011.11.001. Cancer Cell. 2011. PMID: 22094248
-
Metastasis: Signalling in transit.Nat Rev Cancer. 2011 Dec 15;12(1):4. doi: 10.1038/nrc3193. Nat Rev Cancer. 2011. PMID: 22169976 No abstract available.
References
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. - PubMed
-
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

