Rapid versus standard intravenous rehydration in paediatric gastroenteritis: pragmatic blinded randomised clinical trial
- PMID: 22094316
- PMCID: PMC3219422
- DOI: 10.1136/bmj.d6976
Rapid versus standard intravenous rehydration in paediatric gastroenteritis: pragmatic blinded randomised clinical trial
Abstract
Objective: To determine if rapid rather than standard intravenous rehydration results in improved hydration and clinical outcomes when administered to children with gastroenteritis.
Design: Single centre, two arm, parallel randomised pragmatic controlled trial. Blocked randomisation stratified by site. Participants, caregivers, outcome assessors, investigators, and statisticians were blinded to the treatment assignment.
Setting: Paediatric emergency department in a tertiary care centre in Toronto, Canada.
Participants: 226 children aged 3 months to 11 years; complete follow-up was obtained on 223 (99%). Eligible children were aged over 90 days, had a diagnosis of dehydration secondary to gastroenteritis, had not responded to oral rehydration, and had been prescribed intravenous rehydration. Children were excluded if they weighed less than 5 kg or more than 33 kg, required fluid restriction, had a suspected surgical condition, or had an insurmountable language barrier. Children were also excluded if they had a history of a chronic systemic disease, abdominal surgery, bilious or bloody vomit, hypotension, or hypoglycaemia or hyperglycaemia.
Interventions: Rapid (60 mL/kg) or standard (20 mL/kg) rehydration with 0.9% saline over an hour; subsequent fluids administered according to protocol.
Primary outcome: clinical rehydration, assessed with a validated scale, two hours after the start of treatment.
Secondary outcomes: prolonged treatment, mean clinical dehydration scores over the four hour study period, time to discharge, repeat visits to emergency department, adequate oral intake, and physician's comfort with discharge. Data from all randomised patients were included in an intention to treat analysis.
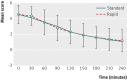
Results: 114 patients were randomised to rapid rehydration and 112 to standard. One child was withdrawn because of severe hyponatraemia at baseline. There was no evidence of a difference between the rapid and standard rehydration groups in the proportions of participants who were rehydrated at two hours (41/114 (36%) v 33/112 (30%); difference 6.5% (95% confidence interval -5.7% to 18.7%; P=0.32). The results did not change after adjustment for weight, baseline dehydration score, and baseline pH (odds ratio 1.8, 0.90 to 3.5; P=0.10). The rates of prolonged treatment were similar (52% rapid v 43% standard; difference 8.9%, 21% to -5%; P=0.19). Although dehydration scores were similar throughout the study period (P=0.96), the median time to discharge was longer in the rapid group (6.3 v 5.0 hours; P=0.03).
Conclusions: There are no relevant clinical benefits from the administration of rapid rather than standard intravenous rehydration to haemodynamically stable children deemed to require intravenous rehydration. Trail registration Clinical Trials NCT00392145.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Intravenous rehydration in paediatric gastroenteritis.BMJ. 2011 Nov 17;343:d7083. doi: 10.1136/bmj.d7083. BMJ. 2011. PMID: 22094317 No abstract available.
-
Rapid rehydration is not better than standard IV hydration in dehydrated pediatric patients with gastroenteritis.J Pediatr. 2012 May;160(5):885-6. doi: 10.1016/j.jpeds.2012.01.066. J Pediatr. 2012. PMID: 22516335 No abstract available.
References
-
- National Collaborating Centre for Women’s and Children’s Health. Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. National Institute for Health and Clinical Excellence, 2009. - PubMed
-
- Bender BJ, Ozuah PO. Intravenous rehydration for gastroenteritis: how long does it really take? Pediatr Emerg Care 2004;20:215-8. - PubMed
-
- National Patient Safety Agency. Alert No 22, ref: NPSA/2007/22. 2007. www.nrls.npsa.nhs.uk/resources/?EntryId45=59809.
-
- Holliday MA, Friedman AL, Wassner SJ. Extracellular fluid restoration in dehydration: a critique of rapid versus slow. Pediatr Nephrol 1999;13:292-7. - PubMed
-
- Nager AL, Wang VJ. Comparison of ultrarapid and rapid intravenous hydration in pediatric patients with dehydration. Am J Emerg Med 2010;28:123-9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
