An NF-κB-independent and Erk1/2-dependent mechanism controls CXCL8/IL-8 responses of airway epithelial cells to cadmium
- PMID: 22094458
- PMCID: PMC3262857
- DOI: 10.1093/toxsci/kfr310
An NF-κB-independent and Erk1/2-dependent mechanism controls CXCL8/IL-8 responses of airway epithelial cells to cadmium
Abstract
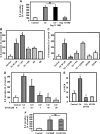
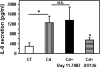
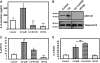
Airway epithelial cells in the lung are the first line of defense against pathogens and environmental pollutants. Inhalation of the environmental pollutant cadmium has been linked to the development of lung cancer and chronic obstructive pulmonary disease, which are diseases characterized by chronic inflammation. To address the role of airway epithelial cells in cadmium-induced lung inflammation, we investigated how cadmium regulates secretion of interleukin 8 (IL-8) by airway epithelial cells. We show that exposure of human airway epithelial cells to subtoxic doses of cadmium in vitro promotes a characteristic inflammatory cytokine response consisting of IL-8, but not IL-1β or tumor necrosis factor-alpha. We also found that intranasal delivery of cadmium increases lung levels of the murine IL-8 homologs macrophage inflammatory protein-2 and keracinocyte-derived chemokine and results in an influx of Gr1+ cells into the lung. We determined that inhibition of the nuclear factor-κB (NF-κB) pathway had no effect on cadmium-induced IL-8 secretion by human airway epithelial cells, suggesting that IL-8 production was mediated through an NF-κB-independent pathway. Mitogen-activated protein kinases (MAPKs) are often involved in proinflammatory signaling. Cadmium could activate the main MAPKs (i.e., p38, JNK, and Erk1/2) in human airway epithelial cells. However, only pharmacological inhibition of Erk1/2 pathway or knockdown of the expression of Erk1 and Erk2 using small interfering RNAs suppressed secretion of IL-8 induced by cadmium. Our findings identify cadmium as a potent activator of the proinflammatory cytokine IL-8 in lung epithelial cells and reveal for the first time the role of an NF-κB-independent but Erk1/2-dependent pathway in cadmium-induced lung inflammation.
Figures







References
-
- Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–1321. - PubMed
-
- Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. - PubMed
-
- Bauer S, Muller T, Hamm S. Pattern recognition by toll-like receptors. Adv. Exp. Med. Biol. 2009;653:15–34. - PubMed
-
- Chambers RC, Laurent GJ, Westergren-Thorsson G. Cadmium inhibits proteoglycan and procollagen production by cultured human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1998;19:498–506. - PubMed
-
- Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 2007;178:6504–6513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

