Role of antibiofilm-antimicrobial agents in controlling device-related infections
- PMID: 22094553
- PMCID: PMC3251652
- DOI: 10.5301/ijao.5000024
Role of antibiofilm-antimicrobial agents in controlling device-related infections
Abstract
Objectives: To assess the effects of N-acetylcysteine (NAC) on organism viability in planktonic and biofilm phases, biofilm thickness, and extracellular polysaccharide content.
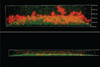
Methods: We performed time-kill curves and broth macrodilution assays of bacterial and fungal clinical isolates with varying concentrations of NAC. We also created in vitro bacterial biofilms, incubated them with NAC or control, and then stained with propidium iodide and FITC-labeled concanavalin A. We measured biofilm thickness, number of non-viable cells, and fluorescent intensity as a marker of extracellular matrix via a confocal laser scanning microscope. All experiments were conducted in triplicate. Tested organisms included methicillin-sensitive and -resistant Staphylococcus aureus (MSSA, MRSA), S. epidermidis, vancomycin-resistant Enterococcus faecalis (VRE), Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae, Candida albicans and C. krusei.
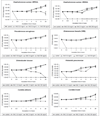
Results: NAC 80 mg/ml was uniformly bactericidal (>99.9% reduction) against all tested bacteria with no recoverable organisms after 30 minutes of incubation, but was fungistatic against candida species. Minimum inhibitory and bactericidal concentrations of NAC ranged from 5-10 mg/ml. Biofilm thickness was significantly decreased in NAC-treated biofilms for all organisms except VRE. The number of non-viable cells in NAC-treated Gram-positive biofilms was increased (p<0.05 for MRSA and VRE). NAC-treated Gram-negative biofilms had scant cellularity and lacked complex 3-dimensional structures that were characteristic of controls. Fluorescent intensity was similar in the experimental and control arms.
Conclusions: NAC is bactericidal against clinically relevant and drug-resistant bacteria and also leads to biofilm disruption. NAC has the potential for use as a novel agent for prevention or treatment of biofilm-related infections.
Conflict of interest statement
Conflict of Interest: S.A. does not have any conflicts of interest.
Figures



References
-
- Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993 Aug;168(2):400–407. - PubMed
-
- Aslam S. Effect of antibacterials on biofilms. Am J Infect Control. 2008 Dec;36(10):S175, e179–e111. - PubMed
-
- Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Pilot trial of N-acetylcysteine and tigecycline as a catheter-lock solution for treatment of hemodialysis catheter-associated bacteremia. Infect Control Hosp Epidemiol. 2008 Sep;29(9):894–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

