In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization
- PMID: 22095157
- PMCID: PMC3265711
- DOI: 10.1007/s11302-011-9271-6
In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization
Abstract
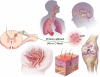
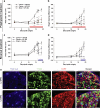
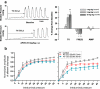
Treating pain by inhibiting ATP activation of P2X3-containing receptors heralds an exciting new approach to pain management, and Afferent's program marks the vanguard in a new class of drugs poised to explore this approach to meet the significant unmet needs in pain management. P2X3 receptor subunits are expressed predominately and selectively in so-called C- and Aδ-fiber primary afferent neurons in most tissues and organ systems, including skin, joints, and hollow organs, suggesting a high degree of specificity to the pain sensing system in the human body. P2X3 antagonists block the activation of these fibers by ATP and stand to offer an alternative approach to the management of pain and discomfort. In addition, P2X3 is expressed pre-synaptically at central terminals of C-fiber afferent neurons, where ATP further sensitizes transmission of painful signals. As a result of the selectivity of the expression of P2X3, there is a lower likelihood of adverse effects in the brain, gastrointestinal, or cardiovascular tissues, effects which remain limiting factors for many existing pain therapeutics. In the periphery, ATP (the factor that triggers P2X3 receptor activation) can be released from various cells as a result of tissue inflammation, injury or stress, as well as visceral organ distension, and stimulate these local nociceptors. The P2X3 receptor rationale has aroused a formidable level of investigation producing many reports that clarify the potential role of ATP as a pain mediator, in chronic sensitized states in particular, and has piqued the interest of pharmaceutical companies. P2X receptor-mediated afferent activation has been implicated in inflammatory, visceral, and neuropathic pain states, as well as in airways hyperreactivity, migraine, itch, and cancer pain. It is well appreciated that oftentimes new mechanisms translate poorly from models into clinical efficacy and effectiveness; however, the breadth of activity seen from P2X3 inhibition in models offers a realistic chance that this novel mechanism to inhibit afferent nerve sensitization may find its place in the sun and bring some merciful relief to the torment of persistent discomfort and pain. The development philosophy at Afferent is to conduct proof of concept patient studies and best identify target patient groups that may benefit from this new intervention.
Figures



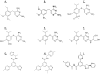
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

