Meta-analysis of phase III trials of docetaxel alone or in combination with chemotherapy in metastatic breast cancer
- PMID: 22095437
- PMCID: PMC3258394
- DOI: 10.1007/s00432-011-1091-0
Meta-analysis of phase III trials of docetaxel alone or in combination with chemotherapy in metastatic breast cancer
Abstract
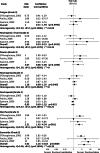
Purpose: Whether combination chemotherapy offers an advantage over sequential therapy in metastatic breast cancer (MBC) is still an unsettled issue. Polychemotherapy regimens containing taxanes has been shown to increase overall survival (OS), time to tumor progression (TTP), and overall response rate (ORR) when compared with regimens that did not contain a taxanes, while taxane-based doublets have a statistically significant benefit over single-agent taxane only for progression-free survival. However, the term "taxanes" generally includes both paclitaxel and docetaxel, drugs with different clinical activity. Aim of this work is to compare OS, TTP, and ORR in patients with MBC receiving docetaxel alone or in combination with chemotherapy using a formal meta-analysis.
Methods: We performed a systematic review of all published trials comparing docetaxel alone or in combination with other chemotherapeutic agents in MBC.
Results: Three randomized clinical trials including 1,313 patients were retrieved. A significant reduction of risk ratio was found in TTP (P ≤ 0.0001) but not in OS (P = 0.48) or ORR (P = 0.10) for patients treated with a chemotherapy agent plus docetaxel compared with docetaxel alone. Treatment with docetaxel alone is associated with a lower incidence of grade 3 diarrhea and stomatitis (diarrhea, P = 0.011; stomatitis, P = 0.0004).
Conclusion: Combination chemotherapy regimens with docetaxel show a statistically significant advantage for TTP, but not for OS and ORR in MBC. This review confirms that it is unlikely that any single agent or combination chemotherapy regimen will emerge as superior in MBC, due to its heterogeneous nature.
Figures
References
-
- Ardavanis A, Kountourakis P, Kyriakou F, Malliou S, Mantzaris I, Garoufali A, Yiotis I, Scorilas A, Baziotis N, Rigatos G (2008) Trastuzumab plus paclitaxel or docetaxel in HER-2-negative/HER-2 ECD-positive anthracycline- and taxane-refractory advanced breast cancer. Oncologist 13(4):361–369. doi:10.1634/theoncologist.2007-0207 - DOI - PubMed
-
- Beslija S, Bonneterre J, Burstein HJ, Cocquyt V, Gnant M, Heinemann V, Jassem J, Kostler WJ, Krainer M, Menard S, Petit T, Petruzelka L, Possinger K, Schmid P, Stadtmauer E, Stockler M, Van Belle S, Vogel C, Wilcken N, Wiltschke C, Zielinski CC, Zwierzina H (2009) Third consensus on medical treatment of metastatic breast cancer. Ann Oncol 20(11):1771–1785. doi:10.1093/annonc/mdp261 - DOI - PubMed
-
- Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, Gelman R, Winer EP (2007) Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer 110(5):965–972. doi:10.1002/cncr.22885 - DOI - PubMed
-
- Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Luck HJ, Mackey JR, Nabholtz JM, Paridaens R, Biganzoli L, Jassem J, Bontenbal M, Bonneterre J, Chan S, Basaran GA, Therasse P (2008) Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol 26(12):1987–1992. doi:10.1200/jco.2007.10.8407 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical