Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection
- PMID: 22095545
- PMCID: PMC3361752
- DOI: 10.1164/rccm.201105-0924OC
Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection
Abstract
Rationale: Fosfomycin/tobramycin for inhalation (FTI), a unique, broad-spectrum antibiotic combination, may have therapeutic potential for patients with cystic fibrosis (CF).
Objectives: To evaluate safety and efficacy of FTI (160/40 mg or 80/20 mg), administered twice daily for 28 days versus placebo, in patients greater than or equal to 18 years of age, with CF, chronic Pseudomonas aeruginosa (PA) airway infection, and FEV(1) greater than or equal to 25% and less than or equal to 75% predicted.
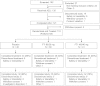
Methods: This double-blind, placebo-controlled, multicenter study assessed whether FTI/placebo maintained FEV(1) % predicted improvements achieved following a 28-day, open-label, run-in course of aztreonam for inhalation solution (AZLI).
Measurements and main results: A total of 119 patients were randomized to FTI (160/40 mg: n = 41; 80/20 mg: n = 38) or placebo (n = 40). Mean age was 32 years and mean FEV(1) was 49% predicted at screening. Relative improvements in FEV(1) % predicted achieved by the AZLI run-in were maintained in FTI groups compared with placebo (160/40 mg vs. placebo: 6.2% treatment difference favoring FTI, P = 0.002 [primary endpoint]; 80/20 mg vs. placebo: 7.5% treatment difference favoring FTI, P < 0.001). The treatment effect on mean PA sputum density was statistically significant for the FTI 80/20 mg group versus placebo (-1.04 log(10) PA colony-forming units/g sputum difference, favoring FTI; P = 0.01). Adverse events, primarily cough, were consistent with CF disease. Respiratory events, including dyspnea and wheezing, were less common with FTI 80/20 mg than FTI 160/40 mg. No clinically significant differences between groups were reported for laboratory values.
Conclusions: FTI maintained the substantial improvements in FEV(1) % predicted achieved during the AZLI run-in and was well tolerated. FTI is a promising antipseudomonal therapy for patients with CF.
Figures

References
-
- Gibson RL, Burns JL, Ramsey BW. State of the art: pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–951 - PubMed
-
- Cystic Fibrosis Foundation Patient Registry 2008 annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2009
-
- Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr 1997;131:809–814 - PubMed
-
- Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol 2010;45:363–370 - PubMed
-
- Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010;303:2386–2392 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

