Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease
- PMID: 22095588
- PMCID: PMC3295906
- DOI: 10.1002/hep.24793
Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease
Abstract
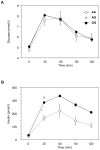
The polymorphism, KLF6-IVS1-27A, in the Krüppel-like factor 6 (KLF6) transcription factor gene enhances its splicing into antagonistic isoforms and is associated with delayed histological progression of nonalcoholic fatty liver disease (NAFLD). To explore a potential role for KLF6 in the development of insulin resistance, central to NAFLD pathogenesis, we genotyped KLF6-IVS1-27 in healthy subjects and assayed fasting plasma glucose (FPG) and insulin sensitivities. Furthermore, we quantified messenger RNA (mRNA) expression of KLF6 and glucokinase (GCK), as an important mediator of insulin sensitivity, in human livers and in liver tissues derived from a murine Klf6 knockdown model (DeltaKlf6). Klf6 overexpression studies in a mouse hepatocyte line were utilized to mechanistically link KLF6 with Gck promoter activity. KLF6-IVS1-27Gwt (i.e., less KLF6 splicing) was associated with stepwise increases in FPG and insulin and reduced hepatic insulin sensitivity. KLF6 binds to the liver-specific Gck promoter and activates a GCK promoter-reporter, identifying GCK as a KLF6 direct transcriptional target. Accordingly, in DeltaKlf6 hepatocytes Gck expression was reduced and stable transfection of Klf6 led to up-regulation of Gck. GCK and KLF6 mRNAs correlate directly in human NAFLD tissues and immunohistochemistry studies confirm falling levels of both KLF6 and GCK in fat-laden hepatocytes. In contrast to full-length KLF6, splice variant KLF6-SV1 increases in NAFLD hepatocytes and inversely correlates with glucokinase regulatory protein, which negatively regulates GCK activity.
Conclusion: KLF6 regulation of GCK contributes to the development of hepatic insulin resistance. The KLF6-IVS1-27A polymorphism, which generates more KLF6-SV1, combats this, lowering hepatic insulin resistance and blood glucose.
Copyright © 2011 American Association for the Study of Liver Diseases.
Conflict of interest statement
There is no conflict of interest to disclose
Figures


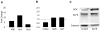


References
-
- Farrell GC. The liver and the waistline: Fifty years of growth. J Gastroenterol Hepatol. 2009;24 (Suppl 3):S105–118. - PubMed
-
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
