Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth
- PMID: 22095729
- PMCID: PMC3974272
- DOI: 10.1161/CIRCRESAHA.111.250126
Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth
Abstract
Rationale: A well-developed coronary collateral circulation improves the morbidity and mortality of patients following an acute coronary occlusion. Although regenerative medicine has great potential in stimulating vascular growth in the heart, to date there have been mixed results, and the ideal cell type for this therapy has not been resolved.
Objective: To generate induced vascular progenitor cells (iVPCs) from endothelial cells, which can differentiate into vascular smooth muscle cells (VSMCs) or endothelial cells (ECs), and test their capability to stimulate coronary collateral growth.
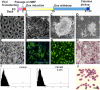
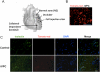
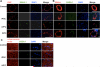
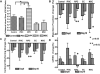
Methods and results: We reprogrammed rat ECs with the transcription factors Oct4, Klf4, Sox2, and c-Myc. A population of reprogrammed cells was derived that expressed pluripotent markers Oct4, SSEA-1, Rex1, and AP and hemangioblast markers CD133, Flk1, and c-kit. These cells were designated iVPCs because they remained committed to vascular lineage and could differentiate into vascular ECs and VSMCs in vitro. The iVPCs demonstrated better in vitro angiogenic potential (tube network on 2-dimensional culture, tube formation in growth factor reduced Matrigel) than native ECs. The risk of teratoma formation in iVPCs is also reduced in comparison with fully reprogrammed induced pluripotent stem cells (iPSCs). When iVPCs were implanted into myocardium, they engrafted into blood vessels and increased coronary collateral flow (microspheres) and improved cardiac function (echocardiography) better than iPSCs, mesenchymal stem cells, native ECs, and sham treatments.
Conclusions: We conclude that iVPCs, generated by partially reprogramming ECs, are an ideal cell type for cell-based therapy designed to stimulate coronary collateral growth.
Figures



Comment in
-
Reprogrammed endothelial cells: cell therapy for coronary collateral growth?Circ Res. 2012 Jan 20;110(2):192-4. doi: 10.1161/CIRCRESAHA.111.261495. Circ Res. 2012. PMID: 22267835 No abstract available.
References
-
- Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. - PubMed
-
- Marban E, Cheng K. Heart to heart: The elusive mechanism of cell therapy. Circulation. 2010;121:1981–1984. - PubMed
-
- Suuronen EJ, Hazra S, Zhang P, Vincent R, Kumarathasan P, Zhang Y, Price J, Chan V, Sellke FW, Mesana TG, Veinot JP, Ruel M. Impairment of human cell-based vasculogenesis in rats by hypercholesterolemia-induced endothelial dysfunction and rescue with L-arginine supplementation. J Thorac Cardiovasc Surg. 2010;139:209–216. e202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

