Regeneration of the heart
- PMID: 22095736
- PMCID: PMC3377117
- DOI: 10.1002/emmm.201100175
Regeneration of the heart
Abstract
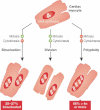
The death of cardiac myocytes diminishes the heart's pump function and is a major cause of heart failure, one of the dominant causes of death worldwide. Other than transplantation, there are no therapies that directly address the loss of cardiac myocytes, which explains the current excitement in cardiac regeneration. The field is evolving in two important directions. First, although endogenous mammalian cardiac regeneration clearly seems to decline rapidly after birth, it may still persist in adulthood. The careful elucidation of the cellular and molecular mechanisms of endogenous heart regeneration may therefore provide an opportunity for developing therapeutic interventions that amplify this process. Second, recent breakthroughs have enabled reprogramming of cells that were apparently terminally differentiated, either by dedifferentiation into pluripotent stem cells or by transdifferentiation into cardiac myocytes. These achievements challenge our conceptions of what is possible in terms of heart regeneration. In this review, we discuss the current status of research on cardiac regeneration, with a focus on the challenges that hold back therapeutic development.
Copyright © 2011 EMBO Molecular Medicine.
Figures


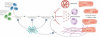

References
-
- Adler CP, Friedburg H. Myocardial DNA content, ploidy level and cell number in geriatric hearts: post-mortem examinations of human myocardium in old age. J Mol Cell Cardiol. 1986;18:39–53. - PubMed
-
- Andersen DC, Andersen P, Schneider M, Jensen HB, Sheikh SP. Murine “cardiospheres” are not a source of stem cells with cardiomyogenic potential. Stem Cells. 2009;27:1571–1581. - PubMed
-
- Astorri E, Chizzola A, Visioli O, Anversa P, Olivetti G, Vitali-Mazza L. Right ventricular hypertrophy-a cytometric study on 55 human hearts. J Mol Cell Cardiol. 1971;2:99–110. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

