Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice
- PMID: 22095841
- PMCID: PMC3319338
- DOI: 10.1002/hep.24783
Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice
Abstract
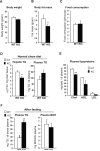
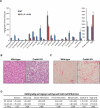
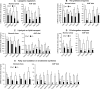
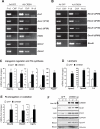
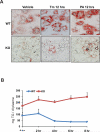
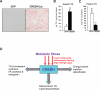
cAMP responsive element-binding protein, hepatocyte specific (CREBH), is a liver-specific transcription factor localized in the endoplasmic reticulum (ER) membrane. Our previous work demonstrated that CREBH is activated by ER stress or inflammatory stimuli to induce an acute-phase hepatic inflammation. Here, we demonstrate that CREBH is a key metabolic regulator of hepatic lipogenesis, fatty acid (FA) oxidation, and lipolysis under metabolic stress. Saturated FA, insulin signals, or an atherogenic high-fat diet can induce CREBH activation in the liver. Under the normal chow diet, CrebH knockout mice display a modest decrease in hepatic lipid contents, but an increase in plasma triglycerides (TGs). After having been fed an atherogenic high-fat (AHF) diet, massive accumulation of hepatic lipid metabolites and significant increase in plasma TG levels were observed in the CrebH knockout mice. Along with the hypertriglyceridemia phenotype, the CrebH null mice displayed significantly reduced body-weight gain, diminished abdominal fat, and increased nonalcoholic steatohepatitis activities under the AHF diet. Gene-expression analysis and chromatin-immunoprecipitation assay indicated that CREBH is required to activate the expression of the genes encoding functions involved in de novo lipogenesis, TG and cholesterol biosynthesis, FA elongation and oxidation, lipolysis, and lipid transport. Supporting the role of CREBH in lipogenesis and lipolysis, forced expression of an activated form of CREBH protein in the liver significantly increases accumulation of hepatic lipids, but reduces plasma TG levels in mice.
Conclusion: All together, our study shows that CREBH plays a key role in maintaining lipid homeostasis by regulating the expression of the genes involved in hepatic lipogenesis, FA oxidation, and lipolysis under metabolic stress. The identification of CREBH as a stress-inducible metabolic regulator has important implications in the understanding and treatment of metabolic disease.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures


References
-
- Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1–26. - PubMed
-
- Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. - PubMed
-
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088227/DK/NIDDK NIH HHS/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- DK090313/DK/NIDDK NIH HHS/United States
- R01 DK090313/DK/NIDDK NIH HHS/United States
- R21 ES017829/ES/NIEHS NIH HHS/United States
- S10RR027926/RR/NCRR NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- S10 RR027926/RR/NCRR NIH HHS/United States
- DK042394/DK/NIDDK NIH HHS/United States
- ES017829/ES/NIEHS NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
