Ptf1a-mediated control of Dll1 reveals an alternative to the lateral inhibition mechanism
- PMID: 22096075
- PMCID: PMC3231770
- DOI: 10.1242/dev.071761
Ptf1a-mediated control of Dll1 reveals an alternative to the lateral inhibition mechanism
Abstract
Neurog3-induced Dll1 expression in pancreatic endocrine progenitors ostensibly activates Hes1 expression via Notch and thereby represses Neurog3 and endocrine differentiation in neighboring cells by lateral inhibition. Here we show in mouse that Dll1 and Hes1 expression deviate during regionalization of early endoderm, and later during early pancreas morphogenesis. At that time, Ptf1a activates Dll1 in multipotent pancreatic progenitor cells (MPCs), and Hes1 expression becomes Dll1 dependent over a brief time window. Moreover, Dll1, Hes1 and Dll1/Hes1 mutant phenotypes diverge during organ regionalization, become congruent at early bud stages, and then diverge again at late bud stages. Persistent pancreatic hypoplasia in Dll1 mutants after eliminating Neurog3 expression and endocrine development, together with reduced proliferation of MPCs in both Dll1 and Hes1 mutants, reveals that the hypoplasia is caused by a growth defect rather than by progenitor depletion. Unexpectedly, we find that Hes1 is required to sustain Ptf1a expression, and in turn Dll1 expression in early MPCs. Our results show that Ptf1a-induced Dll1 expression stimulates MPC proliferation and pancreatic growth by maintaining Hes1 expression and Ptf1a protein levels.
Figures
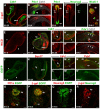
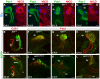
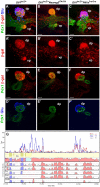
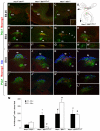
 , P<0.005; by Student’s t-test (compared with wild type); n=3-9. See also supplementary material Fig. S3.
, P<0.005; by Student’s t-test (compared with wild type); n=3-9. See also supplementary material Fig. S3.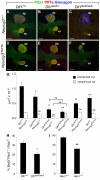

 , P<0.0005; #, P<0.002;
, P<0.0005; #, P<0.002;  , P<0.005; **, P<0.01; *, P<0.05; by Student’s t-test (compared with wild type unless otherwise indicated); n=2-4. ns, not significant.
, P<0.005; **, P<0.01; *, P<0.05; by Student’s t-test (compared with wild type unless otherwise indicated); n=2-4. ns, not significant.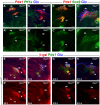

References
-
- Ahnfelt-Ronne J., Jorgensen M. C., Hald J., Madsen O. D., Serup P., Hecksher-Sorensen J. (2007b). An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J. Histochem. Cytochem. 55, 925–930 - PubMed
-
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 - PubMed
-
- Bettenhausen B., Hrabe de Angelis M., Simon D., Guenet J. L., Gossler A. (1995). Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development 121, 2407–2418 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

