P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment
- PMID: 22096244
- PMCID: PMC3286211
- DOI: 10.1182/blood-2011-07-368050
P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment
Erratum in
-
Azab AK, Quang P, Azab F, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119(6):1468-1478.Blood. 2024 Jun 20;143(25):2675. doi: 10.1182/blood.2024024988. Blood. 2024. PMID: 38900471 Free PMC article. No abstract available.
Abstract
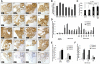
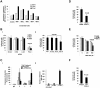
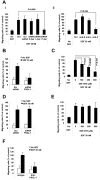
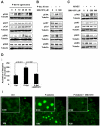
Interactions between multiple myeloma (MM) cells and the BM microenvironment play a critical role in the pathogenesis of MM and in the development of drug resistance by MM cells. Selectins are involved in extravasation and homing of leukocytes to target organs. In the present study, we focused on adhesion dynamics that involve P-selectin glycoprotein ligand-1 (PSGL-1) on MM cells and its interaction with selectins in the BM microenvironment. We show that PSGL-1 is highly expressed on MM cells and regulates the adhesion and homing of MM cells to cells in the BM microenvironment in vitro and in vivo. This interaction involves both endothelial cells and BM stromal cells. Using loss-of-function studies and the small-molecule pan-selectin inhibitor GMI-1070, we show that PSGL-1 regulates the activation of integrins and downstream signaling. We also document that this interaction regulates MM-cell proliferation in coculture with BM microenvironmental cells and the development of drug resistance. Furthermore, inhibiting this interaction with GMI-1070 enhances the sensitization of MM cells to bortezomib in vitro and in vivo. These data highlight the critical contribution of PSGL-1 to the regulation of growth, dissemination, and drug resistance in MM in the context of the BM microenvironment.
Figures



Comment in
-
A new target for myeloma therapy.Blood. 2012 Feb 9;119(6):1325-6. doi: 10.1182/blood-2011-12-394486. Blood. 2012. PMID: 22323404
References
-
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. - PubMed
-
- Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1(7):539–544. - PubMed
-
- Pagnucco G, Cardinale G, Gervasi F. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann N Y Acad Sci. 2004;1028:390–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

