Immunohistochemical and Immunocytochemical Localization of Amylase in Rat Parotid Glands and von Ebner's Glands by Ion Etching-Immunoscanning Electron Microscopy
- PMID: 22096260
- PMCID: PMC3210425
- DOI: 10.1267/ahc.10039
Immunohistochemical and Immunocytochemical Localization of Amylase in Rat Parotid Glands and von Ebner's Glands by Ion Etching-Immunoscanning Electron Microscopy
Abstract
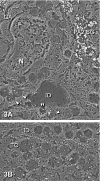
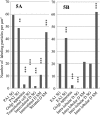
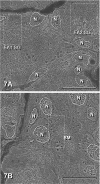
The distribution of amylase in rat parotid glands and von Ebner's glands was examined using ion etching-immunoscanning electron microscopy, which enables both light and electron microscopic observations of identical semi-thin resin sections immunolabeled with anti-α-amylase and immunogold in association with silver enhancement. At the light microscopic level, most acinar secretory granules (SG) and striated duct secretions of parotid glands were strongly stained dark brown. In von Ebner's glands, acinar SG and duct secretions were weakly to strongly stained light to dark brown. At the electron microscopic level, labeling was observed as bright gold-silver particles. The labeling intensity of acinar SG of parotid glands was higher than that of von Ebner's glands. In parotid glands, weak labeling of SG in transitional cells between acini and intercalated ducts, very weak labeling of SG in intercalated ducts, and strong labeling of striated duct secretions were observed. In von Ebner's glands, the secretions and some SG of interlobular ducts were strongly labeled compared to those of intralobular ducts and SG of acini. Less amylase was synthesized in von Ebner's acini compared to parotid acini, whereas von Ebner's ducts may secrete significantly more amylase to modify saliva than parotid ducts.
Keywords: amylase; localization; parotid gland; scanning electron microscopy; von Ebner’s gland.
Figures






Similar articles
-
Immunohistochemical localization and mRNA detection of Rab3D and/or Rab3B in rat von Ebner's glands, parotid gland, pancreas, and liver.Histochem J. 2001 Feb;33(2):71-7. doi: 10.1023/a:1017992012962. Histochem J. 2001. PMID: 11432642 Free PMC article.
-
Ultrastructure of bovine von Ebner's salivary glands.Ann Anat. 1995 Jan;177(1):33-7. doi: 10.1016/S0940-9602(11)80127-5. Ann Anat. 1995. PMID: 7872495
-
Immunohistochemical distribution of immunoglobulins, lactoferrin, and lysozyme in human minor salivary glands.J Oral Pathol. 1984 Apr;13(2):97-104. doi: 10.1111/j.1600-0714.1984.tb01405.x. J Oral Pathol. 1984. PMID: 6425478
-
Functional morphology of myoepithelial cells in the rat salivary glands: A review.J Oral Biosci. 2025 Mar;67(1):100592. doi: 10.1016/j.job.2024.100592. Epub 2024 Nov 29. J Oral Biosci. 2025. PMID: 39615670 Review.
-
Morphological features of the minor salivary glands.Arch Oral Biol. 1999 May;44 Suppl 1:S3-10. doi: 10.1016/s0003-9969(99)90002-x. Arch Oral Biol. 1999. PMID: 10414848 Review.
Cited by
-
Occurrence of gustducin-immunoreactive cells in von Ebner's glands of guinea pigs.Histochem Cell Biol. 2013 Nov;140(5):567-74. doi: 10.1007/s00418-013-1094-9. Epub 2013 Apr 19. Histochem Cell Biol. 2013. PMID: 23604549 Free PMC article.
-
Function of the membrane water channel aquaporin-5 in the salivary gland.Acta Histochem Cytochem. 2012 Oct 31;45(5):251-9. doi: 10.1267/ahc.12018. Epub 2012 Sep 22. Acta Histochem Cytochem. 2012. PMID: 23209334 Free PMC article.
References
-
- Amico F. D., Skarmoutsou E., Imbesi R. M., Sanfilippo S. Early events of secretory granule formation in the rat parotid acinar cell under the influence of isoproterenol. An ultrastructural and lectin cytochemical study. Eur. J. Histochem. 2001;45:169–175. - PubMed
-
- Bendayan M. Protein A gold microscopic immunocytochemistry: methods, applications and limitations. J. Electron Microsc. Tech. 1984;1:243–270.
-
- Field R. B., Hand A. R. Secretion of lingual lipase and amylase from rat lingual serous glands. Am. J. Physiol. 1987;253:G217–225. - PubMed
-
- Field R. B., Spielman A. I., Hand A. R. Purification of lingual amylase from serous glands of rat tongue and characterization of rat lingual amylase and lingual lipase. J. Dent. Res. 1989;68:139–145. - PubMed
-
- Fujiwara T., Shimizu D., Kon K., Isshiki N., Tsunokuni H., Aoyagi S. A new method for detecting and localizing cell markers endocytosed by fibroblasts in epoxy resin semi-thin sections using scanning electron microscopy combined with energy dispersive X-ray microanalysis after ion-etching. J. Electron Microsc. 2000;49:551–558. - PubMed

