Conjunctival reconstruction with progenitor cell-derived autologous epidermal sheets in rhesus monkey
- PMID: 22096478
- PMCID: PMC3214019
- DOI: 10.1371/journal.pone.0025713
Conjunctival reconstruction with progenitor cell-derived autologous epidermal sheets in rhesus monkey
Abstract
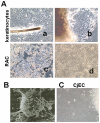
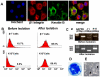
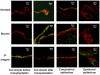
Severe ocular surface diseases are some of the most challenging problems that the clinician faces today. Conventional management is generally unsatisfactory, and the long-term ocular consequences of these conditions are devastating. It is significantly important to find a substitute for conjunctival epithelial cells. This study was to explore the possibility of progenitor cell-derived epidermal sheets on denuded amniotic membrane to reconstruct ocular surface of conjunctiva damaged monkeys. We isolated epidermal progenitor cells of rhesus monkeys by type IV collagen adhesion, and then expanded progenitor cell-derived epidermal sheets on denuded amniotic membrane ex vivo. At 3 weeks after the conjunctiva injury, the damaged ocular surface of four monkeys was surgically reconstructed by transplanting the autologous cultivated epidermal progenitor cells. At 2 weeks after surgery, transplants were removed and examined with Hematoxylin-eosin staining, Periodic acid Schiff staining, immunofluorescent staining, scanning and transmission electron microscopy. Histological examination of transplanted sheets revealed that the cell sheets were healthy alive, adhered well to the denuded amniotic membrane, and had several layers of epithelial cells. Electron microscopy showed that the epithelial cells were very similar in appearance to those of normal conjunctival epithelium, even without goblet cell detected. Epithelial cells of transplants had numerous desmosomal junctions and were attached to the amniotic membrane with hemidesmosomes. Immunohistochemistry confirmed the presence of the conjunctival specific markers, mucin 4 and keratin 4, in the transplanted epidermal progenitor cells. In conclusion, our present study successfully reconstructed conjunctiva with autologous transplantation of progenitor cell-derived epidermal sheets on denuded AM in conjunctival damaged monkeys, which is the first step toward assessing the use of autologous transplantation of progenitor cells of nonocular surface origin. Epidermal progenitor cells could be provided as a new substitute for conjunctival epithelial cells to overcome the problems of autologous conjunctiva shortage.
Conflict of interest statement
Figures

References
-
- Daniels JT, Dart JK, Tuft SJ, Khaw PT. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. - PubMed
-
- Dua HS, Azuara Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. - PubMed
-
- Chiou AG, Florakis GJ, Kazim M. Management of conjunctival cicatrizing diseases and severe ocular surface dysfunction. Surv Ophthalmol. 1998;43:19–46. - PubMed
-
- Corrales RM, Calonge M, Herreras JM, Saez V, Mayo A, et al. Levels of mucin gene expression in normal human conjunctival epithelium in vivo. Curr Eye Res. 2003;27:323–328. - PubMed
-
- Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, et al. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

