Toll-like receptors 2 and 4 regulate the frequency of IFNγ-producing CD4+ T-cells during pulmonary infection with Chlamydia pneumoniae
- PMID: 22096480
- PMCID: PMC3212512
- DOI: 10.1371/journal.pone.0026101
Toll-like receptors 2 and 4 regulate the frequency of IFNγ-producing CD4+ T-cells during pulmonary infection with Chlamydia pneumoniae
Abstract
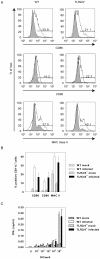
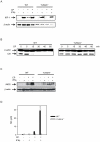
TLR2 and TLR4 are crucial for recognition of Chlamydia pneumoniae in vivo, since infected TLR2/4 double-deficient mice are unable to control the infection as evidenced by severe loss of body weight and progressive lethal pneumonia. Unexpectedly, these mice display higher pulmonary levels of the protective cytokine IFNγ than wild type mice. We show here, that antigen-specific CD4(+) T-cells are responsible for the observed IFNγ-secretion in vivo and their frequency is higher in TLR2/4 double-deficient than in wild type mice. The capacity of TLR2/4 double-deficient dendritic cells to re-stimulate CD4(+) T-cells did not differ from wild type dendritic cells. However, the frequency of CD4(+)CD25(+)Foxp3(+) T-cells was considerably higher in wild type compared to TLR2/4 double-deficient mice and was inversely related to the number of IFNγ-secreting CD4(+) effector T-cells. Despite increased IFNγ-levels, at least one IFNγ-mediated response, protective NO-secretion, could not be induced in the absence of TLR2 and 4. In summary, CD4(+)CD25(+)Foxp3(+) regulatory T-cells fail to expand in the absence of TLR2 and TLR4 during pulmonary infection with C. pneumoniae, which in turn enhances the frequency of CD4(+)IFNγ(+) effector T-cells. Failure of IFNγ to induce NO in TLR2/4 double-deficient cells represents one possible mechanism why TLR2/4 double-deficient mice are unable to control pneumonia caused by C. pneumoniae and succumb to the infection.
Conflict of interest statement
Figures





References
-
- Rodriguez N, Fend F, Jennen L, Schiemann M, Wantia N, et al. Polymorphonuclear Neutrophils Improve Replication of Chlamydia pneumoniae In Vivo upon MyD88-Dependent Attraction. J Immunol. 2005;174:4836–4844. - PubMed
-
- Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. The role of IFN-gamma in the outcome of chlamydial infection. CurrOpinImmunol. 2002;14:444–451. - PubMed
-
- Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, et al. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. JImmunol. 2001;167:6453–6461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

