Identification of residues in the heme domain of soluble guanylyl cyclase that are important for basal and stimulated catalytic activity
- PMID: 22096512
- PMCID: PMC3212528
- DOI: 10.1371/journal.pone.0026976
Identification of residues in the heme domain of soluble guanylyl cyclase that are important for basal and stimulated catalytic activity
Abstract
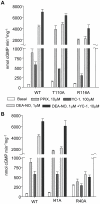
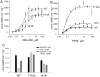
Nitric oxide signals through activation of soluble guanylyl cyclase (sGC), a heme-containing heterodimer. NO binds to the heme domain located in the N-terminal part of the β subunit of sGC resulting in increased production of cGMP in the catalytic domain located at the C-terminal part of sGC. Little is known about the mechanism by which the NO signaling is propagated from the receptor domain (heme domain) to the effector domain (catalytic domain), in particular events subsequent to the breakage of the bond between the heme iron and Histidine 105 (H105) of the β subunit. Our modeling of the heme-binding domain as well as previous homologous heme domain structures in different states point to two regions that could be critical for propagation of the NO activation signal. Structure-based mutational analysis of these regions revealed that residues T110 and R116 in the αF helix-β1 strand, and residues I41 and R40 in the αB-αC loop mediate propagation of activation between the heme domain and the catalytic domain. Biochemical analysis of these heme mutants allows refinement of the map of the residues that are critical for heme stability and propagation of the NO/YC-1 activation signal in sGC.
Conflict of interest statement
Figures


References
-
- Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. - PubMed
-
- Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. - PubMed
-
- Derbyshire ER, Marletta MA. Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol. 2009:17–31. - PubMed
-
- Mergia E, Koesling D, Friebe A. Genetic mouse models of the NO receptor ‘soluble’ guanylyl cyclases. Handb Exp Pharmacol. 2009:33–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

