Atypical scrapie isolates involve a uniform prion species with a complex molecular signature
- PMID: 22096587
- PMCID: PMC3214077
- DOI: 10.1371/journal.pone.0027510
Atypical scrapie isolates involve a uniform prion species with a complex molecular signature
Abstract
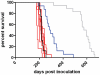
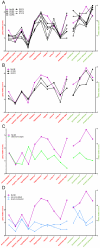
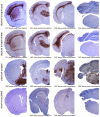
The pathobiology of atypical scrapie, a prion disease affecting sheep and goats, is still poorly understood. In a previous study, we demonstrated that atypical scrapie affecting small ruminants in Switzerland differs in the neuroanatomical distribution of the pathological prion protein (PrP(d)). To investigate whether these differences depend on host-related vs. pathogen-related factors, we transmitted atypical scrapie to transgenic mice over-expressing the ovine prion protein (tg338). The clinical, neuropathological, and molecular phenotype of tg338 mice is similar between mice carrying the Swiss atypical scrapie isolates and the Nor98, an atypical scrapie isolate from Norway. Together with published data, our results suggest that atypical scrapie is caused by a uniform type of prion, and that the observed phenotypic differences in small ruminants are likely host-dependant. Strikingly, by using a refined SDS-PAGE technique, we established that the prominent proteinase K-resistant prion protein fragment in atypical scrapie consists of two separate, unglycosylated peptides with molecular masses of roughly 5 and 8 kDa. These findings show similarities to those for other prion diseases in animals and humans, and lay the groundwork for future comparative research.
Conflict of interest statement
Figures


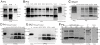

References
-
- Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. - PubMed
-
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. - PubMed
-
- Schneider K, Fangerau H, Michaelsen B, Raab WH. The early history of the transmissible spongiform encephalopathies exemplified by scrapie. Brain Res Bull. 2008;77:343–355. - PubMed
-
- Foster JD, Hope J, Fraser H. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet Rec. 1993;133:339–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

