Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium tuberculosis
- PMID: 22096616
- PMCID: PMC3215101
- DOI: 10.1021/ml200157f
Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium tuberculosis
Abstract
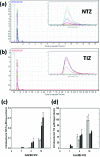
Nitazoxanide (Alinia(®)), a nitro-thiazolyl antiparasitic drug, kills diverse microorganisms by unknown mechanisms. Here we identified two actions of nitazoxanide against Mycobacterium tuberculosis (Mtb): disruption of Mtb's membrane potential and pH homeostasis. Both actions were shared by a structurally related anti-mycobacterial compound, niclosamide. Reactive nitrogen intermediates were reported to synergize with nitazoxanide and its deacetylated derivative tizoxanide in killing Mtb. Herein, however, we could not attribute this to increased uptake of nitazoxanide or tizoxanide as monitored by targeted metabolomics, nor to increased impact of nitazoxanide on Mtb's membrane potential or intrabacterial pH. Thus, further mechanisms of action of nitazoxanide or tizoxanide may await discovery. The multiple mechanisms of action may contribute to Mtb's ultra-low frequency of resistance against nitazoxanide.
Figures


Similar articles
-
A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis.Nat Med. 2008 Aug;14(8):849-54. doi: 10.1038/nm.1795. Epub 2008 Jul 20. Nat Med. 2008. PMID: 18641659 Free PMC article.
-
Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):4976-81. doi: 10.1073/pnas.1400390111. Epub 2014 Mar 17. Proc Natl Acad Sci U S A. 2014. PMID: 24639517 Free PMC article.
-
Nitazoxanide.2023 Sep 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2023 Sep 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 29999627 Free Books & Documents. Review.
-
Mycobacterial genes essential for the pathogen's survival in the host.Immunol Rev. 2015 Mar;264(1):319-26. doi: 10.1111/imr.12256. Immunol Rev. 2015. PMID: 25703569 Free PMC article. Review.
-
Design, Synthesis, and Pharmacokinetic Evaluation of O-Carbamoyl Tizoxanide Prodrugs.Med Chem. 2022;18(1):140-150. doi: 10.2174/1573406416666201120102905. Med Chem. 2022. PMID: 33222677
Cited by
-
Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment.Pharmaceutics. 2021 Apr 21;13(5):592. doi: 10.3390/pharmaceutics13050592. Pharmaceutics. 2021. PMID: 33919204 Free PMC article. Review.
-
Biology of antimicrobial resistance and approaches to combat it.Sci Transl Med. 2020 Jun 24;12(549):eaaz6992. doi: 10.1126/scitranslmed.aaz6992. Sci Transl Med. 2020. PMID: 32581135 Free PMC article. Review.
-
New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing.Antimicrob Agents Chemother. 2013 Feb;57(2):996-1005. doi: 10.1128/AAC.01952-12. Epub 2012 Dec 17. Antimicrob Agents Chemother. 2013. PMID: 23254430 Free PMC article.
-
The membrane as a target for controlling hypervirulent Clostridium difficile infections.J Antimicrob Chemother. 2013 Apr;68(4):806-15. doi: 10.1093/jac/dks493. Epub 2012 Dec 21. J Antimicrob Chemother. 2013. PMID: 23264511 Free PMC article.
-
Derivatives of Pyrimidine Nucleosides Affect Artificial Membranes Enriched with Mycobacterial Lipids.Pharmaceutics. 2024 Aug 23;16(9):1110. doi: 10.3390/pharmaceutics16091110. Pharmaceutics. 2024. PMID: 39339148 Free PMC article.
References
-
- Mitchison D. A. The search for new sterilizing anti-tuberculosis drugs. Front. Biosci. 2004, 9, 1059–1072. - PubMed
-
- Nathan C.; Gold B.; Lin G.; Stegman M.; de Carvalho L. P.; Vandal O.; Venugopal A.; Bryk R. A philosophy of anti-infectives as a guide in the search for new drugs for tuberculosis. Tuberculosis 2008, 88Suppl 1S25–33. - PubMed
-
- Koul A.; Arnoult E.; Lounis N.; Guillemont J.; Andries K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. - PubMed
-
- Makarov V.; Manina G.; Mikusova K.; Mollmann U.; Ryabova O.; Saint-Joanis B.; Dhar N.; Pasca M. R.; Buroni S.; Lucarelli A. P.; Milano A.; De Rossi E.; Belanova M.; Bobovska A.; Dianiskova P.; Kordulakova J.; Sala C.; Fullam E.; Schneider P.; McKinney J. D.; Brodin P.; Christophe T.; Waddell S.; Butcher P.; Albrethsen J.; Rosenkrands I.; Brosch R.; Nandi V.; Bharath S.; Gaonkar S.; Shandil R. K.; Balasubramanian V.; Balganesh T.; Tyagi S.; Grosset J.; Riccardi G.; Cole S. T. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 2009, 324, 801–804. - PMC - PubMed
-
- de Carvalho L. P.; Lin G.; Jiang X.; Nathan C. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J. Med. Chem. 2009, 52, 5789–5792. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

