Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control
- PMID: 22098259
- PMCID: PMC3283443
- DOI: 10.1089/ars.2011.4051
Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control
Abstract
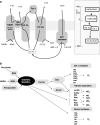
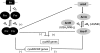
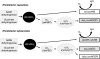
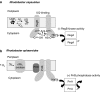
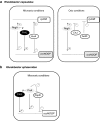
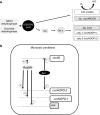
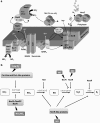
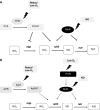
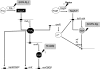
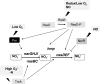
Under a shortage of oxygen, bacterial growth can be faced mainly by two ATP-generating mechanisms: (i) by synthesis of specific high-affinity terminal oxidases that allow bacteria to use traces of oxygen or (ii) by utilizing other substrates as final electron acceptors such as nitrate, which can be reduced to dinitrogen gas through denitrification or to ammonium. This bacterial respiratory shift from oxic to microoxic and anoxic conditions requires a regulatory strategy which ensures that cells can sense and respond to changes in oxygen tension and to the availability of other electron acceptors. Bacteria can sense oxygen by direct interaction of this molecule with a membrane protein receptor (e.g., FixL) or by interaction with a cytoplasmic transcriptional factor (e.g., Fnr). A third type of oxygen perception is based on sensing changes in redox state of molecules within the cell. Redox-responsive regulatory systems (e.g., ArcBA, RegBA/PrrBA, RoxSR, RegSR, ActSR, ResDE, and Rex) integrate the response to multiple signals (e.g., ubiquinone, menaquinone, redox active cysteine, electron transport to terminal oxidases, and NAD/NADH) and activate or repress target genes to coordinate the adaptation of bacterial respiration from oxic to anoxic conditions. Here, we provide a compilation of the current knowledge about proteins and regulatory networks involved in the redox control of the respiratory adaptation of different bacterial species to microxic and anoxic environments.
Figures


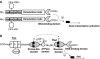



References
-
- Alvarez AF. Georgellis D. In vitro and in vivo analysis of the ArcB/A redox signaling pathway. Methods Enzymol. 2010;471:205–228. - PubMed
-
- Arai H. Kodama T. Igarashi Y. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol Microbiol. 1997;25:1141–1148. - PubMed
-
- Arnoux P. Sabaty M. Alric J. Frangioni B. Guigliarelli B. Adriano JM. Pignol D. Structural and redox plasticity in the heterodimeric periplasmic nitrate reductase. Nat Struct Biol. 2003;10:928–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

